Effect of Soil Chemical and Physical Properties on Cd Mobility Studied by Soil Thin-Layer Chromatography
S. S. AL Oud1 * , M. H. EL-Saeid1 and M.E. Nadeem1
1
Soil Sciences Department,
King Saud University,
Riyadh,
11451
Kingdom of Saudi Arabia
DOI: http://dx.doi.org/10.12944/CWE.9.3.02
Heavy metals contamination threat to the environment is a big concern. Predicting the movement of heavy metals such as Cd in soil is an important goal. In this study the effect of soil chemical and physical properties on Cd mobility were done using a thin layer chromatography (Soil TLC). The soils used in this research have a wide range of organic matter (2.1-29 g/kg), pH value (4.9-8.8), CEC (4.1-99 cmol/kg), bases saturation, iron and aluminum oxides to examines its effect on Cd mobility. The (Rf) values obtained from soil thin layer chromatography ranged from 0.25 to 0.95 and it showed that Cd was slightly mobile in 64 %, moderately mobile in 29 % and very mobile in 7 % of the soils. The Rf indicates that Cd mobility would decrease with increasing amounts of iron oxide fractions; silt % and exchangeable Mg2+ in the soils. Rcd values increased with increasing amounts of the iron oxide fractions (Feo, Fep, and Fed); silt % and exchangeable Mg2+ in the soils. Finally, both Rcd and Rf factors showed Cd mobility to be higher in those soils with a high percentage of sand.
Copy the following to cite this article:
AL Oud S. S, EL-Saeid M. H, Nadeem M. E. A. Effect of Soil Chemical and Physical Properties on Cd Mobility Studied by Soil Thin-Layer Chromatography. Curr World Environ 2014;9 (3). DOI:http://dx.doi.org/10.12944/CWE.9.3.02
Copy the following to cite this URL:
AL Oud S. S, EL-Saeid M. H, Nadeem M. E. A. Effect of Soil Chemical and Physical Properties on Cd Mobility Studied by Soil Thin-Layer Chromatography. Curr World Environ 2014;9(3). Available from: http://www.cwejournal.org/?p=7838
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2014-11-21 |
|---|---|
| Accepted: | 2014-12-09 |
Introducion
Heavy metal contamination is of concern due to release of metals in the environment by numerous factors like the industrial activities and in agricultural soils due to use of fertilizers, pesticides, fungicides and herbicides (Roth, et. al., 2012). Cadmium (Cd) in one of the heavy metals which is most mobile and bioavailable and is of high concern for its ecotoxicity. This has been demonstrated in various studies showing impact of Cd contamination on soil, groundwater, ecosystems and agriculture (Moradi et al., 2005; Keller et al., 2002; Van der Grift and Griffioen,2008). Cd sorption to soil phases mainly depends on soil properties like the pH, soil organic matter and clay content (Streck and Richter, 1997; Deurer and Bottcher, 2007). Cd partitions between solid phases and soil solution affect the sorption and desorption processes which in turn affect the Cd toxicity, mobility, bioavailability and reactivity in environment.
Soil constituents along with the metal hydroxides are indicated to be participating in the sorption and desorption of Cd in soil(Krishnamurti and Naidu, 2003; Calace et al., 2009). Several important factors controlling the distribution of Cd between soil and solutes have been identified. The concentration of competing cations such as Ca or other heavy metals, pH and organic matter content significantly affect the Cd sorption equilibrium (Vega, et. al.,2010). Minimizing the threat of soil and groundwater pollution by accurately predicting the movement and cycling of hazardous contaminants is an ultimate goal. It has been suggested that the soil is the ultimate receptacle of most solid and liquid wastes (Shaheen, 2009).
After studying the movement of some trace elements including Cd in 11 soils, it was reported that the soil texture, surface area, percentage of iron oxides, and pH provide the most useful information for estimating an element's migration (Seguraet al., 2006). It was reported that when the pH value dropped below 6, the mobility of Cd increases as compared with a higher pH value. Moreover, they added that the availability of heavy metals is lower in natural loamy soils than in sandy acidic soils due to precipitation of carbonates and phosphates in the former soil type(Scokat et al., 1983).
Metals may occur in the soil solution in different forms, each having a different mobility and toxicity. Hydrolysis species of Cd do not contribute to total Cd in acidic soils (Lindsay, 1979). Studies done on the chemistry of metals in forest soils of South Sweden have shown a strong correlation between soil solution pH and total Cd (Berggren,1992). Cadmium was shown to be fairly mobile in soils of pH 4.6 to 6.6 and moderately mobile in those between 6.7-7.8 pH units(Fuller, 1977). The mobility of Cd in various natural soils by soil thin-layer chromatography was studies and was found that, the ratio of distance moved by cadmium to the one moved by the developer,Rf, range between 0.14 and 1.0 for soils with a mean of 0.64. It was concluded that Cd was slightly mobile in 27%, moderately mobile in 14%, mobile in 41% and highly mobile in 18% of the soils studied (Sanchez-Cmazano et al., 1993). The pH, sum of the exchangeable bases, exchangeable Ca and Mg, CEC, and clay content have a significant effect on cadmium mobility in soils (Sanchez-Cmazano et al., 1993). It wasreported that the results of complex trace metal (Cd and Zn) interactions with soil components show a higher accumulation capacity for loamy soil than for sandy soil even though they have a similar CEC (Scokart et al., 1983). The addition of organic-rich wastes such as municipal sludges and industrial residues can provide enough organic matter to the soil to bind other toxic heavy metals such as Cd, Zn, and Pb. It was suggested that heavy metals below sewage disposal sites can move downward through the soil column as soluble metal-organic complexes (MaCarthy and Perdue, 1991 , Harrison, et. al., 1999).
It was observed that metal-binding organic substances in waste water or sludge designed for land use may affect the fate of toxic metals. Cd adsorption in the presence of soluble organic ligands, ammonium acetate (Ac), oxalate (Ox), nitrilotriacetate (NTA), and EDTA was modified based on a competition for the metal ions between soil metal-binding sites and added soluble organic materials. In addition when the ligand bond was weaker than metal-soil such as Ox-Cd, adsorption was not affected. However, for ligands capable of strongly binding Cd (NTA and EDTA) out of competing for soil sites, adsorption was reduced due to the formation of non adsorbing complexes (Elliott and Denney 1982). Therefore it has been suggested to avoid the use of effluent containing chelating agents for irrigation (Kirkham, 1977). The importance of organic ligands on the mobility of metals in soil environments should be minimal for acidic solutions and weakly complexing ligands. (Elliott and Denney, 1982).There have been a number of studies involving the collective investigation of soil properties on the Cd sorption and mobility various regression techniques have been implemented to study what properties best describe the effect on soil Cd sorption process (Shaheen, 2009, Vega, et. al., 2010 and Cerqueira, et. al., 2011).
The aim of this research work is to study the effect of different soil characteristics on Cd mobility using soil thin layer chromatography (TLC) and explain the relationship between different components of soil relevant to Cd sorption process and to possibly understand the combining effects of these soil properties in the process.
Material and Methods
Particle size distribution was determined by the pipette method after removing the cementing agents and dispersed by sodium pyrophosphate. The cation exchange capacities ( CEC) values were determined using the displacement method, using NaOAc buffered at pH 8.2 with Na as the index cation. Additionally, NH4OAc extractable bases, namely Ca, Mg, K, and Na, were measured employing an IL Video12 atomic absorption spectrophotometer and flame emission analyses. Extractable iron and aluminum contents of the studied soils were determined according to Blakemore et al., (1987). The poorly crystalline, Feo and Alo of the Fe and Al bearing minerals were extracted, using acid ammonium oxalate in the dark. For the total (poorly and well crystalline) oxides, hydroxides and oxyhydroxides of Fe and Al (Fed and Ald) the soils were extracted using dithionite-citrate-bicarbonate (DCB) buffer. Sodium pyrophosphate was used to extract the organically bound Fe and Al, (Fep and Alp). The concentration of the inorganic poorly crystalline Fe fraction, Fep – Fep, was calculated from the difference between poorly crystalline and organically bound oxides (Evans and Wilson, 1985). Soil pH was measured potentiometrically in a 1:1 water to soil suspensions by using a combined pH-electrode.
Preparation of the Soil Plates
The Cadmium mobility was studied by means of soil thin layer chromatography(Soil TLC). The Soil samples were ground in a mortar and sieved through a 160-μm nylon mesh sieve to obtain samples with a small and nearly homogeneous particle size. A soil water slurry of solid:solution ratio of 1:2 (7.5 g of soil and 15 ml of distilled water) was then prepared. The plates then were coated with water slurry of the soil (0.5 mm thickness) over each of the 20 x 5 cm plates with aid of an ordinary TLC applicator. The selected plates, which have an even soil thickness distribution, were then air dried at room temperature and relative humidity of 70% in a desiccating chamber.
Soil TLC Procedures
The plates were marked with two horizontal lines at different distances from the base (3 and 13 cm) so that the standard development distance of 10 cm was used in all the plates. A 5 μl aliquot of cadmium nitrate solution (Cd (NO3)2 0.1 M) was applied as spot on the base line of triplicate TLC plates in a single application with the micro pipette. The drops were placed at a distance of 3 cm from the bottom of the plates.
The loaded plates were then developed by ascending chromatographic tank containing distilled water as solvent. The development was done for 1-3 hour depending on soil sample textures. Then, the plates were washed for 13 cm from the baseline and allowed to dry at room temperature. The dried plates were sprayed with 0.05 % dithizone in CCl4 in order to detect Cd as orange spots of Cd-dithizone.
Cadmium mobility was measured visually as the frontal Rf (cm) value (mobility) using the following relationship:
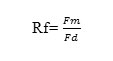
Where Fm is frontal distance moved by the metal and Fd is the one moved by the developer solution.
|
|
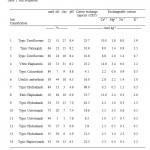
Table 1: Soil Properties |
|
|
Table 2: Soil Properties |
Results and Discussion
Soil Properties
The fourteen soils investigated in this research are characterized by the parameters given in Tables 1 and 2. The soils range in particle size distribution and texture from sand to silty clay.
They exhibited a range of pH values from strongly acidic (pH of 4.9) to strongly alkaline (pH of 8.8), and a wide range of CEC from very low of 4.1 cmol/kg to very high of 99.0 cmol/kg.
Mobility in Relation to Soil Properties
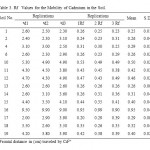
Table 3, shows the cadmium mobility Rf values which were derived from the chromatograph obtained for the different soils, which are considered to be a measure of mobility in thin layer chromatography (TLC). The frontal
Rf values was estimated using the following relationship
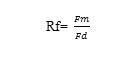
Where Fm is frontal distance moved by the metal and Fd is the one moved by the developers solution.
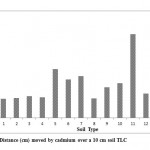
The Rf values obtained ranged between 0.25 and 0.95 (cm). The results suggest that Cd mobility in these soils was highly variable. Because the soil TLC technique has only recently begun to be used for estimating metal mobility such as Cd in soils, and also with the lack of sufficient published data in this field, there is no mobility classification according to Rf except the one proposed by Helling and Turner (1968) for pesticide mobility in soils. The cadmium mobility classifications in the soils studied according to Hellingand Turner (1968) are shown in Table 4. Based on this classification cadmium was slightly mobile in 64%, moderately mobile in 29 % and very mobile in 7% of the soils investigated. The distance moved by cadmium (Fig. 1) was the highest in the soil with a high sand percentage (soil 16) and the lowest for those of high silt and/or clay percentages (soil 1). In general soils with high percentages of sand (soil 10, 12, and 15) fall in the moderately mobile class.
|
|
Figure 1: Distance (cm) moved by |
There was a significant correlation at the 0.05 probability level between Rf values and sand percentage. There were also a significant negative correlation (p < 0.05) between silt %,clay %, exchangeable (Ca and K) soil contents and the Rf values. The other soil properties including pH, CEC, and organic carbon content, where not significant at (p < 0.05).
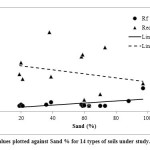
The variability in the cadmium mobility in the soils is mostly accounted for by sand (r = 0.63), followed by silt %; (r = -0.59); the exchangeable Ca(r = -0.55); clay % (r = -0.55)and the exchangeable K (r = -0.49). The Rf values below 0.35 were generally measured for soils with relatively a higher percentage of clay and silt than the rest of the soils studied. Both clay and silt percentages showed a significant correlation with measured Rf values. The Soils that have Rf> 0.35 or soils classified as moderately mobile with respect to cadmium movements, shared either a higher sand % (soil 12, and 15), and /or lower pH values as the case of soil 19. Only one soil, soil 16, classified as very mobile with regards to cadmium movements has the highest amount of sand (94%) and the lowest clay content of (1%) . The Rf values of the soils were plotted as a function of sand % in soils and shown in (Fig. 2). The plots show that the higher the sand %, the higher will be the mobility of Cd in soils. The divalent cation, Ca2+, was negatively correlated with Rf (p < 0.05), which could be a result of competition between Cd2+ and exchangeable Ca2+ for the sorbing sites. McBride et al, (1981), found the cadmium retention capacity of soils to be dependent significantly on the exchangeable Ca2+ content of soils. It was found the competition of cadmium with exchangeable cations to decrease in the following order Al < Ca < K < Na. The competition of Cd with these cations was also found in this study to decrease in the order of Ca < K < Na < Mg which is consistence with the variation of the correlation coefficients of Rf with the amounts of these elements in the soils (Matos et al., 2001, Benaissa and Benguella, 2004). The correlation between Rf values and soil pH was insignificant and negative which indicates at least that increasing the soil pH will decease Rf and cadmium mobility in soils. Soil organic carbon content and CEC showed a negative and insignificant correlation with Rf, which suggests that these factors can be
|
|
Figure 2: Rf values plotted against Sand |
|
|
Table 3: Rf Values for the Mobility |
Table 4: Cadmium Cobility in Soils.
|
aClass |
Rf |
Mobility |
Soil No. |
Soil (%) |
|
2 |
0.10-.34 |
Slightly mobile |
1, 2, 4, 6, 11, 13, 14, 17, 18 |
64 |
|
3 |
0.35-0.64 |
Moderately mobile |
10, 12, 15, 19 |
29 |
|
4 |
0.65-0.89 |
Mobile |
0 |
0 |
|
5 |
0.90-1.0 |
Very mobile |
16 |
7 |
aClassification according to Helling and Turner (1968). important in limiting cadmium mobility to a lesser degree than divalent cations specially Ca2+ or clay and silt contents. The correlation between Rf values and iron and aluminum was insignificant. The contents of iron and aluminum oxides in the soils showed that they played an insignificant role in cadmium mobility.
Assessment of Groundwater Contamination Potential
The mobility of Cd also was evaluated by means of the cadmium retardation factor, Rcd.
The retardation factor Rcd, represents the ratios of the average linear velocity of groundwater (Ï) to that of dissolved Cd (Ïs).
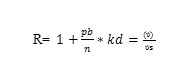
Where pb the soil bulk density, n is the soil porosity, and the kd is the empirical distribution coefficient for sorbing Cd which is the solid phase concentration of Cd divided by the dissolved Cd concentration. Also, the Rcd value can be calculated from, fw, which is the form of a compound in the water solution in a volume containing both solid and water as follows
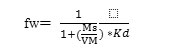
Where Ms is mass of soil used (0.5 g), and VM is the volume of the solution used (25 ml). The Rcd is equal to 1/fw. The kd factor is usually expressed in units of ml/g, derived as follows:
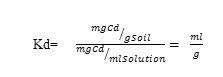
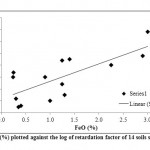
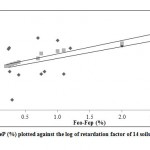
The Kd values for soils studied were obtained by mean of batch adsorption experiment at fixed total Cd load of 12.8 ppm on order to calculate the retardation factor values of Cd (Rcd) . The Rcd values of the soils studied at fixed total Cd load of 12.8 ppm and at their natural pH varied from 2.9 to 33.42. The Rcd values for the soils studied were found to be increasing with increasing pH and also to differ from soil to soil. In order to determine the influence of soil properties on the cadmium movement, the simple correlation coefficient between the cadmium retardation factor Rcd and soil properties was conducted. There is significant correlations between Rcd values and Feo, Fe0-Fep, and silt (p < 0.01); and Fed at (p< 0.05); Fep and the exchangeable Mg2+ at (p < 0.1). Also there is a significant, though negative correlation between Rcd and sand % at ( p < 0.05 ). The Rcd values increase with increasing amounts of the iron oxide fractions (Feo, Fep, and Fed); silt% and exchangeable Mg2+ in the soils (Figure 3 and 4).
|
Figure 3: FeO (%) plotted against the log of |
|
|
Figure 4: FeO-FeP (%) plotted against the log of |
The quantities of extractable aluminum oxide fractions in the soils show a negative but not significant correlation with measured Rcd. Soils with a high percentage of sand seem to favor cadmium mobility over retention or low Rcd values and high Rf values (Fig.2). Both Rcd and Rf factors were significantly correlated (p < 0.05) with sand and silt percentages. Rcd increases rapidly with pH and is consistently somewhat greater for soils with higher iron contents.. The Rcd increased with increasing quantities of either poorly crystalline inorganic or organically bound irons in soils.
Conclusion
The Rf values obtained ranged from 0.25 to 0.95 which suggested that Cd mobility in these soils is highly variable. Cadmium mobility was found to be slightly mobile in 64%, moderately mobile in 29% and very mobile in 7% of the soils investigated. The variability in the cadmium mobility in the soils is mostly accounted for by the sand % (r = 0.63), followed by the silt % ; (r = -0.59); the exchangeable Ca(r = -0.55); the clay % (r = -0.55) and the exchangeable K (r = -0.49). Rcd values increased with increasing amounts of the iron oxide fractions (Feo, Fep, and Fed); silt % and exchangeable Mg2+ in the soils. Finally, both Rcd and Rf factors showed Cd mobility to be higher in those soils with a high percentage of sand.
Acknowledgment
This project was supported by King Saud University, Deanship of Scientific Research, College of Food & Agriculture Sciences, Research Center.
References
1. Roth, E., Mancier, V., Fabre, B. Adsorption of cadmium on different granulometric soil fractions: Influence of organic matter and temperature, Geoderma, 189–190, 133-143. (2012).
2. Moradi, A., Abbaspour, K.C., Afyuni, M. Modelling field-scale cadmium transport below the root zone of a sewage sludge amended soil in an arid region in Central Iran. Journal of ContaminantHydrology 79, 187–206 (2005).
3. Keller, A., Abbaspour, K.C., Schulin, R. Assessment of uncertainty and risk in modeling regional heavy-metal accumulation in agricultural soils. Journal of Environmental Quality 31, 175–187.(2002)
4. Van der Grift, B., Griffioen, J. Modelling assessment of regional groundwater contamination due to historic smelter emissions of heavy metals. Journal of Contaminant Hydrology 96, 48–68 (2008).
5. Streck, T., Richter, J. Heavy metal displacement in a sandy soil at the fieldscale: I. Measurements and parameterization of sorption. Journal ofEnvironmental Quality 26, 49–56.(1997).
6. Deurer, M., Bottcher, J. Evaluation of models to upscale the small scalevariability of Cd sorption in a case study. Geoderma 137, 269–278. (2007).
7. Krishnamurti, G., Naidu, R. Solid–solution equilibria of cadmium in soils. Geoderma 113, 17–30. (2003).
8. Calace, N., Deriu, D., Petronio, B.M., Pietroletti, M. Adsorption isotherms and breakthrough curves to study how humic acids influence heavy metal–soilinteractions. Water Air Soil Pollut. 204, 373–383. (2009).
9. Vega, F.A., Andrade, M.L. CoveloE.F. Influence of soil properties on the sorption and retention of cadmium, copper and lead, separately and together, by 20 soil horizons: Comparison of linear regression and tree regression analyses. Journal of Hazardous Materials, 174, 522–533. (2010).
10. Shaheen, S,M. Sorption and lability of cadmium and lead in different soils from Egypt and Greece. Geoderma, 153, 61–68.(2009).
11. Segura, R.,Arancibia, V.,Zú˜niga, M. C.,Pastén, P.,2006, Distribution of copper, zinc, lead and cadmium concentrations in stream sediments from the Mapocho River in Santiago, Chile, J. Geochem. Explor. 91, 71–80.(2006).
12. Scokart, P. O., Meeus-Verdinne, K. & De Borger, R. Mobility of heavy metals in polluted soils near zinc smelters. Water Air Soil Pollut., 20, 451-63. (1983).
13. Lindsay,W.L. Chemical equilibrium in soils. John Wiley, New York. (1979).
14. Berggren, D. Speciation and mobilization of aluminum and cadmium in Podozols and Cambisols of South Sweden. Water, Air ,and Soil Pollute, 62: 125-126. (1992).
15. Fuller,W.H. Movement of selected metals. Asbestos and cyanide in soil: Application to waste disposal problem. EPA-600/2-77- 020. Solid and Hazardous waste Research Cincinnati, OH. (1977).
16. Sanchez-Camazano. M Sanchez-Martin, M. J. 1993.Adsorption and mobility of cadmium in natural, uncultivated soils. J. Environ. Qual. 22: 737-742. (1991)
17. MaCarthy P, Perdue E.M. Complexation of metal ions by humic substances: fundamental considerations. In: Bolt FH, DeBoot MF, Hayes MHB, McBride MB (eds), Interactions at the Soil Colloid-Soil Solution Interface, pp.469- 489. Kluwer Academic Publishers, Dordrecht. (1991).
18. Harrison, E.Z., McBride, M.B. and Bouldin, D.R. Land application of sewage sludges: an appraisal of the US regulations. Int. J. Environment and Pollution, 11, 1–36. (1999).
19. Elliott, A. H., and Denney, C. M. Soil adsorbtion of cadmium from solutions containing organic ligands. Journal of Environmental Quality, 11, 658-662. (1982).
20. Kirkham, M.B. Elemental composition of sludge-fertilized chrysanthemums. Journal of the American Society for Horticultural Science 102: 352-354. (1977).
21. Cerqueira, B.,Covelo, E. F., Andrade, L., Vega, F. A. The influence of soil properties on the individual and competitive sorption and desorption of Cu and Cd. Geoderma, 162, 20–26. (2011).
22. Blakemore, S.C., P.S. Searle, and B.K. Daly. Methods for chemical analysis of soils. N.Z. Bur, Sci. Rp.80. N.Z. Soil Bureau, Sower Hutt, N.Z.(1987).
23. Evans, L. J. and W.G. Wilson. Extractable Fe, Al, Si and C in B horizons of Podozolic and Brunisolic soils from Ontario. Can. J. of Soil Sci. 65: 489-496. (1985).
24. Helling, C. S., and Tumer, B.C. Pesticide mobility: Determination by soil thin-layer chromatography. Science, 162: 562-563. (1968).
25. Matos A.T., M.P.F. Fontes, L.M. da Costa and M.A. Martinez. Mobility of heavy metals as related to soil chemical and mineralogical characteristics of Brazilian soils. Environmental Pollution. 111: 429-435. (2001).
26. Benaissa, H., and Benguella, B. Effect of anions and cations on cadmium sorption kinetics from aqueous solutions by chitin: experimental studies and modeling. Environmental Pollution, 130, 157-163. (2004).