Bioethanol Production from Enzymatically Hydrolysed Cotton Stalk: One Approach Towards Sustainable Energy Development
Mirza Zaheer Baig1 * and Smita M. Dharmadhikari1
1
Department of Microbiolog,
Government Institute of Science,
Aurangabad (M.S.),
431004
India
DOI: http://dx.doi.org/10.12944/CWE.9.3.46
Copy the following to cite this article:
Baig M. Z, Dharmadhikari S. M. Bioethanol Production from Enzymatically Hydrolysed Cotton Stalk: One Approach Towards Sustainable Energy Development. Curr World Environ 2014;9 (3) DOI:http://dx.doi.org/10.12944/CWE.9.3.46
Copy the following to cite this URL:
Baig M. Z, Dharmadhikari S. M. Bioethanol Production from Enzymatically Hydrolysed Cotton Stalk: One Approach Towards Sustainable Energy Development. Curr World Environ 2014;9(3). Available from: http://www.cwejournal.org/?p=6821
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2014-07-14 |
|---|---|
| Accepted: | 2014-08-14 |
Worldwide increasing energy demand anddecreasing fossil reservoir led to the resurgence in development of alternative fuel, which must be renewable and environmental friendly. Unlike fossil fuel, ethanol is renewable energy source produced through fermentation of sugar. Ethanol can be produced from variety of biomass and among various biomasses; lignocellulosic biomass source is plentiful and economical resource that can serve as source of ethanol production at large scale.1 Lignocellulosic biomass sources include: agricultural wastes, industrial wastes, forestry wastes and municipal solid wastes, etc.2 Cotton stalk which are left behind after the cotton harvest, is one of the example of a lignocellulosic agricultural waste. There are about 32 million hectares of cotton cultivable area across the world and about 10 million hectares in the country.3 Since cotton stalk is a by-product of cotton crop; India has an abundance of this lignocellulosic biomass source. The objective of present study is to evaluate the potential of ethanol production from alkali pre-treated and enzymatically hydrolysed cotton stalk by suing co culture of Saccharomycescerevisiaeand Pachysolentannophilus.
Materials and Methods
Collection of raw material
The cotton (GossypiumhirsutumNHH44) stalkused in this research work was harvested material from the farmer's field of Marathwada region.
Physical pretreatment
The cotton stalk which consist of different unwanted residues were removed mechanically by shredding followed by sundried, debarked, bailed and ground to 1mm particle size with laboratory blender and stored in tightly sealed plastic bags.
Compositional analysis
A major portion of biomass feed stock is made up of carbohydrates, which are polysaccharide in nature. These carbohydrate sub units were quantified by HPLC (Zodiac. Ltd) using laboratory analytical proceure-002(LAP-002), standard protocol of NREL (National Renewable Energy Laboratory).4 The lignin was also determined as per NREL procedure.
Alkaline pretreatment and enzyme hydrolysis
Alkaline pretreatment and enzyme hydrolysis was carried out by following the guideline from previous research studies.5
Alkaline pretreatment
Alkaline pretreatment was performed by treating 2% NaOH at substrate loading of 10% (w/v) and the flask were sterilized for 60 minutes at 121oC. After pretreatment the biomass has been separated from lignified liquor by centrifugation at 10000 rpm for 10 minutes and supernatant (black liquor) was separately collected from each sample for quantitative detection of lignin. The delignified biomass was repeatedly washed with distilled water till to become neutral pH and dried in oven at 60oC and was stored for further studies.6
Enzyme hydrolysis
Enzymatic hydrolysis of pre-treated biomass was carried out using commercial cellulases purchased from Sisco Research Laboratories Pvt. Ltd. Mumbai, India. Pre-treated cotton stalk was incubated with 5% solid loading in 50mM acetate buffer (pH 4.8) with 100 CMC (Caroxy methyl cellulose) unit of enzyme and was incubated at 50oC with 150 rpm for 72 hours. After incubation, the sample was centrifuged in chilled condition at 5000 rpm for 10 minutes and supernatant was collected as fermentation sugar.
Fermentation of enzyme hydrolysate of cotton stalk
Fermentation of detoxified hydrolyzate of cotton stalk was carried out by using Saccharomyces cerevisiae MTCC 36 and Pachysolen tannophilus MTCC 1077 purchased from Microbial Type Culture Collection, IMTECH, Chandigarh, India. Lyophilized culture of Saccharomyces cerevisiae and Pachysolen tannophilus were activated separately on yeast and malt extract (YM) medium. The medium was prepared by adding 0.3% yeast extract, 0.3% malt extract, 0.5% peptone and 1% glucose in distilled water. pH of the medium was adjusted to 6.5.7 The yeast cells were allowed to grow aerobically at 30oC on rotary shaker incubator with 120 rpm for 48 hours or till the culture partially covered the bottom of flask. Completely activated yeast cells were actively transferred to YM agar plates and allowed to grow at 30oC for 48 hours and purity was checked microscopically from isolated colonies.
Inoculum preparation
Inoculum was prepared in detoxified hydrolyzate solution of cotton stalk supplemented with 0.5% yeast extract, 1% peptone and pH was 5.5%. The yeast cells which were harvested by centrifugation were added in inoculum and incubated on rotary shaker incubator with 150 rpm at 30oC for 24 hours8 and grown aerobically to promote healthy growth of yeast cells in hydrolyzate and used as inoculum for fermentation. The volume of inoculum again set to 10% to the total volume used for fermentation. For quantifying the cell mass, One millilitre aliquot from each suspension was taken to performed serial dilution up to 105 and 100 µL of diluted culture was spread-plated on to YM agar plates by adding 0.3% yeast extract, 0.3% malt extract, 0.5% peptone, 1% glucose and 2.5% agar and were incubated at 30oC for 48 hours and yeast colonies were counted to ensure that each time the inoculation stayed at approximately 6.0 × 107cfu/mL corresponding to 10g dry weight/Litre.
Ethanol productions from enzymatic hydrolysate of cotton stalk
The filtrated product obtained from enzymatic hydrolysis of cotton stalk was used as sole carbon source for fermentation and was supplemented with 0.1% (w/v) yeast extract, peptone, NH4Cl, KH2PO4 and 0.05% (w/v) of MgSO4.7H2O, MnSO4, CaCl2.2H2O, FeCl3.2H2O and ZnSO4 in 250 mL Erlenmeyer flasks. Fermentations were performed in semi aerobic mode (250 mL Erlenmeyer flask containing 150 mL of fermentation medium) having pH 5.5 and sterilized at 110oC for 20 minutes.9 The flasks were inoculated with 10% co-culture of Saccharomycescerevisiae and Pachysolentannophilus at concentration of 6% and 4% respectively. The flasks were sealed with aluminium foil and incubated on rotary shaker with 120 rpm for first 24 hours and then kept in static mode at 30oC for 96 hours. Sample was removed from each flask at one time at the interval of 12 hours and analysed for ethanol, residual sugar and cell biomass concentration.
Determination of ethanol, residual sugars and cell growth
Sample obtained during fermentation was transferred to pre weighted centrifuged tube and were centrifuged at 10000 rpm for 10 minutes at 4oC. The supernatant was collected and analysed for concentration of ethanol and residual sugars in broth while pellet was repeatedly washed with distilled water and dry in hot air oven at 60oC till constant weight.10
Analytical method
Analytical tools and methods applied to conduct the study are as follows.
Total reducing sugar
After appropriate dilution the solubilisation of fermentable sugars were determined by DNS (3, 5-dinitrosalicyclic acid) method of Miller.11
Ethanol estimation by Gas Chromatography
After each experiment, part of supernatant was filtered by 0.22 µm cellulose acetate filter and analyzed by Gas Chromatography (Shimadzu Japan). All analysis was carried out according to NREL (National Renewable Energy Laboratory) procedure LAP # 011 using ZB-Wax column (30mm × 0.25mm) with Flame Ionization Detector.12
Statistical analysis
Statistical analysis were carried out in factorial completely randomized design (CRD) by software MAUSTAT developed by department of statistics ofVasantraoNaikMarathwada Agriculture University, Parbhani, Maharashtra, India.
Results and Discussions
Compositional analysis of cotton stalk T
he major chemical composition of cotton stalk is cellulose, hemicellulose and lignin but their concentration varied depending on growing location, harvesting methods as well as analysis procedure.13 Cotton stalk (Gossypiumhirsutum) used in this study was composed of 42% glycan and 22% xylan while other ingredient of hemicellulose was in very small proportion. The lignin content was 24.18%. Our results are harmony with the results reported earlier.6,14,15
Alkaline pretreatment and enzyme hydrolysis
Alkaline pretreatment significantly delignified cotton stalk and increases the sugar concentration in residual pre-treated biomass. Cellulases can provide huge benefits inutilization of biomass in long term because of the possible high glucose yields and opportunity to apply the modern tools of biotechnology to reduce cost.16 The result reported that when cotton stalk powder at substrate loading of 10% (w/v) was subjected to 2% NaOH at 121oC steam explosion in steam sterilizer for 60 minutes was significantly removed lignin of 0.201 g/g of biomass and when this delignified biomass was subjected to enzyme hydrolysis by incubating with 5% solid loading in 50mM acetate buffer (pH 4.8) and exposing 100 CMC (Carboxy Methyl Cellulose) unit of enzyme concentration(per gram of biomass) at 50oC with 150 rpm for 72 hours, yielded 0.49 gram of fermentable sugar per gram of biomasscorresponds to the concentration of 24.5 g/L.5 Finally the obtained enzyme hydrolyzate of cotton stalk was used as sole carbon source for ethanol production.
Fermentation of enzyme hydrolysate of cotton stalk by co-culture of Saccharomyces cerevisiae and Pachysolen tannophilus
The sugar solution obtained from optimized alkaline pre-treated and enzymatically hydrolysed cotton stalk were fermented for analysing the potential of bioethanol production through fixed parameters and outcomes of all experimental setup were evaluated by using three analytical parameters simultaneously including ethanol concentration in the fermentation broth, substrate utilization and growth of cell mass. The reliability of results was checked statistically by passing through ANOVA (analysis of variance). The setup was conducted at 30oC, using 250 mL Erlenmeyer flasks containing 150 mL of fermentation broth (semi aerobic nature) loaded with cotton stalk hydrolysate as sole carbon source having sugar concentration of 24.5 g/L. The fermentation was started with addition of 10% inoculum (6% Saccharomycescerevisiae and 4% Pachysolentannophilus) and were agitated for first 24 hours and then kept in static mode up to 96 hours. Samples were withdrawn from 06 hours onwards followed by at every 12 hours interval of time from separate flask and were analysed.
Resultant data obtained after statistical analysis is presented in Table 1. indicates that, ethanol was not detected in first 6 hours of incubation while sugar consumption and cell mass concentrationgot started at the rate of 18.56% and 2.42 g/L respectively. As for as ethanol production is concern, it commence from 12 hours onwards which gives 0.86 g/L as shown in Fig 1. which corresponds to an yield of 0.017 g/g of native cotton stalk, 0.27 g/g of holocelluloses and 0.35 g/g of fermentable sugar and continuously increases up to 48 hours of incubation and finally maximum ethanol production was recorded at 48 hours which produces 9.56 g/L corresponds to yield 0.191 g/g of biomass, 0.298 g/g of holocelluloses and 0.392 g/g of fermentable sugar, beyond which the ethanol concentration remained constant and show slight fall mainly due to feedback inhibition or catabolic repression. The fermentation efficiency at 48 hours of incubation was recorded as 76.85% while more than 97% sugars of hydrolysate were effectively consumed by yeast cultures as shown in Fig 3. Simultaneously cell mass concentration was also increased up to 36 hours of incubation (12.14 g/L) and after that no significant change was observed. Moreover no ethanol was detected in hydrolysate of pre-treated samples generated in absence of enzyme (control) as no fermentable sugars were available. Interestingly, it was observed that as co-culture was used for fermentation, but no diauxy growth pattern was observed during growth and production. These finding were harmony with results reported earlierduring simultaneous saccharification and fermentation of the alkali-treated cotton stalks resulted in ethanol concentration and ethanol yield was 19.48 g/L and 0.21 g/g of biomass respectively, by using thermo tolerant Pichiakudriavzevii HOP-1.17
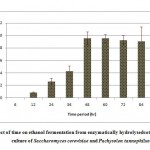 |
Figure 1: Effect of time on ethanol fermentation from enzymatically hydrolysed cotton stalk by co-culture of Saccharomyces cerevisiae and Pachysolen tannophilus Click here to View Figure |
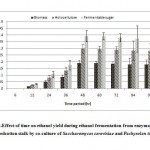 |
Figure 2: Effect of time on ethanol yield during ethanol fermentation from enzymatically hydrolysed cotton stalk by co-culture of Saccharomyces cerevisiae and Pachysolen tannophilus Click here to View Figure |
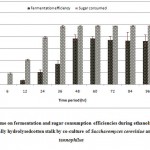 |
Figure 3: Effect of time on fermentation and sugar consumption efficiencies during ethanol fermentation from enzy matically hydroly sedcotton stalk by co-cultureof Saccharomyces cerevisiae and Pachysolen tannophilus Click here to View Figure |
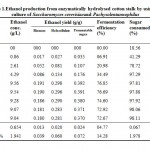 |
Table 1: Ethanol production from enzymatically hydrolysed cotton stalk by using co-culture of Saccharomyces cerevisiaeand Pachysolentannophilus Click here to View table |
Conclusions
Conclusively, the maximum ethanol production from enzyme hydrolysate of cotton stalk was recorded at 48 hours of incubation which gives an ethanol concentration of 9.56 g/L with a yield of 0.191 g/g of biomass, 0.298 g/g of holocelluloses and 0.392 g/g of fermentable sugar. The fermentation and sugar consumption efficiencies were recorded as 76.85% and 97.81% respectively. Ethanol production is affected by variety of factors, including concentration of substrate, cellular activity in co culture environment and reaction conditions such as pH, temperature, time etc. and in this regards this study can serve as a one step towards sustainable energy development and more efforts were needed in terms of process optimization to make the process more feasible.
References
- Dien, B.S., Iten, L.B., and Bothast, R.J. Conversion of corn fiber to ethanol by recombinant E.coli strain FBR3. J IndMicrobiol. 22: 575-581(1999).
- Zaldivar, J., Nielsen, J., and Olsson, L. Fuel ethanol production from lignocellulose: A challenge for metabolic engineering and process integration. ApplMicrobiolBiotechnol. 56: 17-34(2001).
- Kranthi, K.R., Venugopalan, M.V., Sabesh, M., and Yadav, M.S. Vision 2030. Central Institute for Cotton Research, Nagpur (2011). http://www.cicr.org.in/pdf/CICR_VISION_2030.pdf
- Ruiz, R., and Ehrman, T. Determination of carbohydrates in biomass by high performance liquid chromatography. Laboratory analytical procedure no. 002. Golden CO: National Renewable Energy Laboratory(1996).
- MirzaZaheerBaig and Dharmadhikari, S.M.Optimization of pre-treatment and enzymatic hydrolysis of cotton stalk. J Pure and ApplMicrobiol. 6(03): 1437-1441(2012).
- Silverstein, R.A., Chen, Y., Sharma-Shivappa, R.R., Boyette, M.D., and Osborn, J. A. A comparison of chemical pre-treatment methods for improving saccharification of cotton stalks. Bioresour Technol. 98: 3000-3011(2007).
- Chandel, A.K, Narasu, A.K., Rudravaram, R., Pogaku, R., and Rao, L.V. Bioconversion of De-Oiled Rice Bran (DORB) hemi cellulosic hydrolyzate into ethanol by Pichiastipites NCM3499 under optimized condition. Int Jfood Eng. 5(1): 1-12(2009).
- Srilekha Yadav, K., Naseeruddin, S., Prashanthi, G.S., Sateesh, L., and Rao, L.V. Bioethanol fermentation of concentrated rice straw hydrolyzate using co-culture of Saccharomyces cerevisiae and Pichia stipites. Bioresour Technol. 102(11): 6473-6478(2011).
- Pasha, C., Kuhad, R.C., and Rao, L.V. Strain improvement of thermo tolerant Saccharomyces cerevisiae VS3 strain for better utilization of lignocellulosic substrates. J ApplMicrobiol. 103: 1480-1489(2007).
- Oberoi, H.S., Vadlani, P.V. Madl, R.L., Saida, L., and Abeykoon, J.P. Ethanol production from orange peels: two-stage hydrolysis and fermentation studies using optimized parameters through experimental design. J Agric Food Chem. 58: 3422-3429(2010).
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal chem. 31: 426-428(1959).
- David, T.W. Determination of ethanol concentration in biomass to ethanol fermentation supernatant by Gas Chromatography. NREL, LAP-011(1994).
- Agblevor, F.A., Batz, S., and Trumbo, J. Composition and ethanol production potential of cotton gin residues. ApplBiochemBiotechnol. 105: 219-230(2003).
- Ververis, C., Georghiou, K., Christodoulakis, N., Santas, P., and Santas, R. Fiber dimensions, lignin and cellulose contents of various plant materials and their suitability for paper production. Ind crop Prod.19(3): 245-254(2004).
- Binod, P., kuttiraja, M., Archana, M., Janu, K.U., Sindhu, R., Sukumaran, R.K., and Pandey, A. High temperature pre-treatment and hydrolysis of cotton stalk for producing sugars for bioethanol production. Fuel. 92: 340-345(2012).
- Himmel, M.E., Ruth, M.F., and Wyman, C.E. Cellulases for commodity products from cellulosic biomass. CurrOpinBiotechnol. 10: 358-364(1999).
- Kaur, U., Oberoi, H.S., Bhargav, V.K., Sharma-Shivappa, R.R., and Dhaliwal, S.S. Ethanol production from alkali and ozone treated cotton stalk using thermo tolerant Pichiakudriavzevii HOP-1. Ind crop prod. 37, 219-226(2012).






