Larval Morphology And Development Of Tree Frog Polypedates Teraiensis (Dubois, 1987)
Dulumoni Tamuly1 * and Mithra Dey1
DOI: http://dx.doi.org/10.12944/CWE.9.1.25
Copy the following to cite this article:
Tamuly D, Dey M. Larval Morphology And Development Of Tree Frog Polypedates Teraiensis (Dubois, 1987). Curr World Environ 2014;9(1) DOI:http://dx.doi.org/10.12944/CWE.9.1.25
Copy the following to cite this URL:
Tamuly D, Dey M. Larval Morphology And Development Of Tree Frog Polypedates Teraiensis (Dubois, 1987). Curr World Environ 2014;9(1). Available from: http://www.cwejournal.org/?p=5675
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2013-11-15 |
|---|---|
| Accepted: | 2014-02-14 |
Anurans having a biphasic life cycle, breed in a variety of water bodies ranging from lentic to lotic water bodies. Anuran tadpoles exhibit structural diversities that are associated with their habitat, foraging behaviour and predator avoidance. The tree frog Polypedates teraiensis is a common rhacophorid breeding between April to August in north east India and is known to deposit eggs in the foam nest. There are at least six species of Polypedates currently recognized in north-eastern India (Chakravarty et al. 2011). However, little is known about the larval biology of these species from this region. The present study describes the oral disc, various morphometric features of the tadpoles, size and stage at hatching and duration of life history (post hatching) from Cachar district, Assam, north-east India.
Materials and Methods
Between April to September, 2011 and 2012 several foam nests of Polypedates teraiensis were sighted in manmade tanks in Assam University campus constructed for water storage for construction work. The foam nests were found adhering to the wall of the tanks slightly above the water surface. Some of the foam nests were brought to the laboratory and kept in aquaria with pond water for hatching. Tadpole rearing was done in the laboratory at the temperature 26-33ÌŠ C. The clutch sizes were recorded. Data are based on three clutches. Various developmental stages were fixed in 10% formaldehyde at periodic interval and duly measured. Tadpoles were staged according to Gosner (1960). Sampling was repeated for two successive years and the average data for three different cycles are presented herein. Tadpoles were fed on fish food and algae collected from the pond. Morphometric measurements of various developmental stages were taken using vernier calliper. These include BL, TL, BD, BW, T, TH, BTMH, IO, IN, SO and SN. Abbreviations and definitions are in accordance with Altig and McDiarmid (1999). Description of oral apparatus and labial tooth row formula (LTRF) is in accordance with Altig (1970).
Abbreviations
BL-Body length, TL-Total length, BW-Body width, BD-Body depth, I-O- Interorbital distance, I-N-Internarial distance, S-O-Snout orbit distance, S-N-Snout naris distance, T-Tail length, BTMH- Basal tail musculature height, TH-Tail height.
Results
The frog is a seasonal breeder, breeding only during the monsoon. Depending on the rainfall the breeding season extended from April to September. During the study, the tanks were filled with rain water and the bottom was found to be covered with debris material, decaying leaves and mud. There was no other tadpoles found in the tanks, but the tanks were inhabited by other adult anurans such as Fejervarya sp. and Euphlyctis cyanophlyctis. Insect fauna was also abundant in the tank. The foam nests were found 4-5 inch above the water body adhering to the wall, some floating on the surface of water .The nest were collected from the tank and brought to the laboratory for rearing. It took 1-2 days for hatching after the collection. The number of hatching per nest ranged between 100 to150. The hatchlings measured about 7.8 mm in total length and were at stage 22 (Gosner stage). The life history (post hatching) was completed within 42 days. Hours and days taken for development, lowest, highest and average length of different developmental stages are presented in Table 1.
Table 1: Developmental Stages of Polypedates teraiensis
|
Sr no. |
Stages |
Corresponding stages of Polypedates teraiensis |
Time for development |
Lowest length(mm) |
Highest length(mm) |
Average length(mm) |
|
1 |
Fertilized egg |
- |
0 |
- |
- |
- |
|
2 |
External gill |
22-24 |
96 hrs |
6.8 |
12 |
9.43 |
|
3 |
Feeding |
25 |
7 days |
10.5 |
12.7 |
11.61 |
|
4 |
Hind limb bud development |
26-30 |
12-21 days |
14.2 |
26 |
20.05 |
|
5 |
Toe differentiation and development |
31-39 |
25-32 days |
25 |
45 |
33.69 |
|
6 |
Well developed hind limb |
40 |
35 days |
40 |
44 |
41.3 |
|
7 |
Forelimb visible |
41 |
36 days |
40 |
47 |
43.5 |
|
8 |
Both limbs |
42-45 |
37-39 days |
40 |
45 |
42.2 |
|
9 |
Froglet |
46 |
42 days |
15 |
17 |
16.2 |
Tadpole Morphology
Body is oval, snout slightly rounded and depressed, eyes lateral in position. Nostrils dorsal, nearer to snout than eyes. Spiracle single sinistral, position lateral, vent dextral. Dorsal fin height is greater than the ventral fin. Both fin gradually tapering towards the pointed tip. Black spot is present all over the body and tail. Ventral side of the body is not pigmented and transparent at the abdomen region. Hence the intestinal spiracle is clearly visible through the transparent abdominal wall. Morphometric measurement of various developmental stages is presented in Table 2.
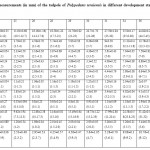 |
Table 2: Morphometric measurements (in mm) of the tadpole of Polypedates teraiensis in different development stages. N=10 (X±SD; range in parenthesis). Click here to View table |
Oral disc:
Mouth anteroventral, marginal papillae are biserially arranged. Teeth blunt and are not same in height. Lower jaw ‘v’ shaped and jaw sheath finely serrated. Upper jaw arch shaped with a weak median convexity, both jaw sheaths edged with black. Submarginal papillae present. Disc emarginate, labial papillae and beak disappearing by stage 42
LTRF
4(2-4)/3(1). First row of the upper labium continuous whereas the 2nd, 3rd, and 4th rows are interrupted. Innermost row of lower labium slightly interrupted whereas the two other rows are continuous (Fig: 4).
 |
Figure 1: Foam with Eggs Fig 2: Gosner stage 23 Fig 3: Gosner Stage 31 Fig 4: Mouth part at Stage 38 Click here to View figure |
Coloration
The tadpoles are light brown in colour with brown pigments all over the body and tail portion. Fin transparent.
Discussion
P. teraiensis breeds in temporary pools, tanks that are filled by rain water during monsoon. The life history of this frog (post hatching) was completed with in 42 days. Relatively short period of development is characteristic of tropical species which have to take advantage of transitional aquatic habitat during the monsoons (Heyer 1973). This short period of development in this species is characteristic to take advantage, as it allows the larvae to metamorphose quickly and escape desiccation as the tanks dry up. Sheridan (2008) reported larval life (post hatching) of Polypedates leucomystax in 42 days from Sakaeerat, northeastern Thailand and the froglet measured about 19.4 mm. This developmental time is similar to the present report. Chakravarty et al. (2011) reported from Assam that metamorphosis was completed in P. teraiensis in 58 days. Polypedates maculatus completed development and metamorphosis in 55 days in Bhubaneswar (Hejmadi and Dutta, 1988). Girish and Saidapur (1999) reported the metamorphosis time of Polypedates maculatus as 60 days. Saidapur (2001) reported the larval duration of Polypedates maculates in 50-70 days and the size of metamorphosis is 21-23 mm. Metamorphosis (i.e., stages 42-46) lasts 6 days in the present study. This is similar to other available data on the duration of metamorphosis in P. maculatus and R. arboreus which undergo metamorphosis in five days (Iwasawa and Kawasaki, 1979; Mohanty-Hejmadi and Dutta, 1988). Downie et al. (2004) who studied timing of metamorphosis in 14 taxonomically and ecologically diverse species from Trinidad (Daudin,1802) reported metamorphosis ranging from 2.0 to 7.3 days. Whereas Sekar (1990) reported metamorphosis duration in R. malabaricus as 12 days. Chakravarty et.al (2011) reported metamorphosis duration of 9 days in P. teraiensis.
The foam nest is essential for development of this rhacophorid species, when eggs were removed from the foam nest before hatching the embryo did not develop further (Chakravarty et.al, 2011). This is also observed in the present study. The foam nest protects the eggs and embryo from predators and desiccation (Heyer, 1969; Downie, 1988) and also protected from thermal damage, as white foam nest reflects heat (Gorzula, 1977). Deposition of eggs away from water protects the early stages of the embryos (Mohanty and Dutta, 1988). The tadpole of small temporary ponds have been reported to spend more time in feeding and develop faster than tadpoles from larger permanent ponds, where the larvae spend more time hiding from predators and develop more slowly (Peltzer and Lajmanovich 2004).
The clutch size for P. teraiensis ranged between100-150 in the present study. Chakravarty et al. (2011) reported from Assam that the clutch size for P. teraiensis consists about 100 eggs. Mohanty and Dutta (1988) reported for P. maculates, the number of eggs ranged from 275-719, where as Girish and Saidapur (1999) found the number of hatchling per nest ranged between 210-448 in P. maculatus. The present data is similar to the earlier published data and difference may be due to temperature and humidity variation in the present study area.
The embryonic development takes place within the foam nest, the tadpole in stage 21 stays within the nest and drops into water at stage 22. At stage 22 the larvae are very delicate and tail fins become transparent. The external gills get reduced and finally covered with development of operculum at stage 25. At stage 41 almost fully developed forelimbs are seen concealed beneath the transparent skin .The pigmentation is visible at stage 22 on the dorsal side of the body. The keratodont rows are quite distinct at stage 25 and LTRF formula is 4(2-4)/3(1). With the emergence of forelimbs at stage 42, the keratodonts and jaw sheaths have completely disappeared. In the present study the life cycle duration of 42 days is similar to an earlier study conducted in the present location (unpublished, Dey, 1997) where it was completed in 40 days.
Conclusion
The duration of development and metamorphosis of anurans has been found to vary from species to species. The metamorphosis is completed in 58 days in P. teraiensis, 55days in Polypedates maculates, 94 days in Rana cyanophlyctis, 68 days in Rhacophorus malabaricus, 64 days in Hyla annectans, 60-61 days in Polypedates leucomystax, 59-60 days in Rhacophorus bipunctatus, 35-50 days in Bufo melanostictus as reported by earlier workers.
Based on the present findings, it can be concluded that foam nest is essential for the development of this rhacophorid species. The newly hatched larvae are very delicate with a large yolk sac and external gills. The keratodont rows are quite distinct at stage 25 and LTRF formula is 4(2-4)/3(1). At stage 42 the keratodonts and jaw sheaths have completely disappeared. The life history (Post hatching) of Polypedates teraiensis was completed within 42 days during the month of April to September under the favourable climatic factors. These findings can be used in planning the conservation of the frog under its natural habitats
Acknowledgements
The authors are grateful to the Department of Ecology and Environmental Science, Assam University, Silchar where the work was carried out and to the field assistant who helped in the field collection.
References
- Gosner K. L., A simplified table for staging anuran embryos and larvae with notes of identification. Herpetologica, 16: 183-190 (1960)
- Chakravarty P., Bordoloi S., Grosjean S.,Ohler A., and Borkotoki A., Tadpole morphology and table of developmental stages of Polypedates teraiensis (Dubois,1987). Alytes, 27(3): 85-115 (2011)
- Altig R. & McDiarmid R.W., Body plan. Development and morphology. In: R. W. McDiarmid& R.Altig (ed.), Tadpoles: the biology of anuran larvae, Chicago, University of Chicago Press: 24-51 (1999)
- Altig R., A key to the tadpoles of Continental United States and Canada. Herpetologica. 26: 180-207 (1970)
- Heyer R .W., Ecological interaction of frog larvae at a seasonal tropical location in Thailand. J. Herpetol 7(4): 337-361(1973)
- Sheridan J. A., Ecology and behavior of Polypedates leucomystax (Anura: Rhacophoridae) in northeast Thailand. Herp. Rev., 39: 165-169 (2008)
- Monhanty H. P. and Dutta S. K., Life history of the common tree frog, Polypedates maculatus (Gray, 1834) (Anura: Rhacophoridae). J. Bombay nat Hist. Soc., 85: 512-517 (1988)
- Girish S. and Saidapur S.K., Mating and nesting behavior and early development in the tree frog Polypedates maculatus. Current Science, 76: 91-92 (1999)
- Saidapur S. K., Behavioral ecology of anuran tadpoles: The Indian Scenario. Proc. Indian natn Sci Acad, 6: 311-322 (2001)
- Iwasawa H. and Kawasaki N., Normal stages of development of the Japanese green frog, Rhacophorus arboreus. Jap. J. Herp., 8: 22-35 (1979)
- Downie J. R., Bryce R. and Smith J., Metamorphic duration: an under-studied variable in frog life histories. Biol. J. linn. Soc., 83: 261-272 (2004)
- Sekar A. G., Observation on the developmental stages of tadpoles of Malabar gliding frog, Rhacophorus malabaricus Jerdon, 1870 (Anura: Rhacophoridae). J. Bombay nat. Hist. Soc., 87: 223-226 (1990)
- Heyer W.R., The adaptive ecology of the species groups of the genus Leptodactylus (Amphibia, Leptodactylidae). Evolution, 23: 421-428 (1969)
- Downie J. R., Functions of the foam in the foam-nesting leptodactylid Physalaemus pustulosus. Herp. J., 1: 302-307 (1988)
- Gorzula S., Foam nesting in leptodactylids: a possible function. Brit. J.Herp., 5:657-659 (1977)
- Peltzer P. M. and Lajmanovich R.C., Anuran tadpole assemblages in riparian areas of the middle parana river, Argentina. Biodiversity and Conservation, 13:1833-1842 (2004)






