Adsorption of Pb(II) from aqueous solution on Ailanthus Excelsa tree bark
V.H Waghmare1 * and U.E.Chaudhari 1
1
Department of Chemistry,
Mahatma Fule Arts, Commerce and Sitaramji Chaudhari Science Mahavidyalya Warud,
Amravati,
India
DOI: http://dx.doi.org/10.12944/CWE.8.3.21
Copy the following to cite this article:
Waghmare V. H, Chaudhari U. E. Adsorption of Pb(II) from aqueous solution on Ailanthus Excelsa tree bark. Curr World Environ 2013;8(3) DOI:http://dx.doi.org/10.12944/CWE.8.3.21
Copy the following to cite this URL:
Waghmare V. H, Chaudhari U. E. Adsorption of Pb(II) from aqueous solution on Ailanthus Excelsa tree bark. Curr World Environ 2013;8(3). Available from: http://www.cwejournal.org/?p=5415
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2013-11-04 |
|---|---|
| Accepted: | 2013-12-10 |
At present lead pollution is considered a worldwide problem because this metal is commonly detected in several industrial wastewaters.1 Undoubtedly, industrial waste based adsorbents offer a great promise for commercial purposes. Solid wastes are a vexing societal problem mandating attention to recycling. Recycled product quality is not always high or recycle may not be feasible. However, conversion of solid wastes into effective low-cost adsorbents for wastewater treatments could decrease costs for removing lead. Water used in industry creates a wastewater that has a potential hazard for our environment because of introducing various contaminants such as heavy metals into soil and water resources. Heavy metal ions are nowadays among the most important pollutants in surface and ground water.2 The safe and effective disposal of industrial wastewater is thus a challenging task for industrialists and environmentalists. The important toxic metals are Cd, Zn, Pb and Ni. Nowadays, with the exponential increase in population, measures for controlling heavy metal emissions into the environment are essential. Lead causes many serious disorders like, anemia, kidney disease, nervous disorders, and even death3
There are numerous methods currently employed to removal of metals from aqueous environment. Some of these methods are chemical precipitation and sludge separation, chemical oxidation or reduction, ion exchange, reverse osmosis, membrane separation, electro chemical treatment, evaporation and adsorption.Among all these, adsorption is the most promising technique and economically feasible alternative for metal removal.
Adsorption method offers the advantages of low operating cost and minimizing secondary pollution. Plant material is easily available and relatively; inexpensive an investigation of its use as a adsorbent seems most appropriate Earlier researchers used different plant materials such as Sawdust of Dalbergiasissoo, babhul Bark , Mangifera indica (mango), coconut fibers and Madicago sativa (alfalfa) for metal removal from wastewater In the present work, the Pb (II) ions adsorption capacity of Ailanthus Excelsa tree bark (AETB) was studied by a batch technique. The effect of pH, concentration of Pb(II) ions, contact time and adsorbent dose on percentage of adsorption has also been investigated.
Material and Methods
Preparation of Adsorbent
Ailanthus Excelsa (AETB) tree bark was collected from a local farm. It was cut in to small segment and dried in sunlight until almost all the moisture evaporated. Then it was ground to get desired particle size of 100 to 200 micron. It was then soaked 2 hours in 0.1M NaOH solution to remove the lignin content. Excess alkalinity was then removed by neutralizing with 0.1 N HCl. The AETB was then washed several times with distilled water till the washings are free from color and turbidity. The washed AETB was oven dried at 200 C for 24 hrs and stored for the study.
Preparation of Solutions
All the reagents used were of AR grade. Pb (II) solution Stock Pb (II) ions solution (1000 mg/L) was prepared by dissolving 0.331 gm of A.R. grade Pb(NO3)2 in 1000 ml distilled water. The solutions of lower concentrations were prepared by dilution of appropriate volume of stock solution. 0.1M Sodium Thiosulphate solution was preapared by dissolving 1.5810gm of A.R. grade Sodium Thiosulphate in 1000 ml distilled water. Dithizone 50ml 3% dithizone solution in choloroform
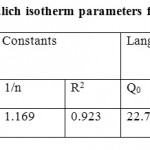 |
Table 1: Langmuir and Freundlich isotherm parameters for Pb (II) ions uptake by AETB Click here to View table |
Results and Discussion
Effect of pH
The pH of feed solution was examined from solutions at different pH, covering a range of 2.0–6.0. There was continuous increase in percentage removal with increase in pH and reached 53.5 % at pH 6. The increase in percentage removal may be attributed to higher degree of ionization of metal ion at higher pH and the reduced competition of H+ ions with the metal ions for adsorption sites. The removal of Pb (II) ions decreases rapidly bellow pH 4. At pH< 4.0, H+ ions compete with Pb (II) ions for the surface of the adsorbent which would hinder Pb(II) ions from reaching the binding sites of the adsorbent caused by the repulsive forces. At pH grater than 4. For this reason the maximum pH value was selected to be 4.5.
Effect of Contact Time
The effect of contact time on the amount of Pb (II) ions adsorbed was investigated using 10 and 20 mg/L initial concentration of Pb (II) ions with 0.5 and 1 gm/ (AETB) at pH 4.5. The effect of contact time and metal concentration on the percent removal of Pb (II) ions by AETB ispresented. The results indicate removal of Pb (II) ions increases with increase in contact time and equilibrium was attained in about 360 min. The extent of removal of Pb(II) by AETB was found to increase, reach a maximum value with increase in contact time.
Effect of Adsorbent Dose
The effect of adsorbent dose on the removal of Pb(II) ions was investigated using 10 mg/L of initial Pb (II) concentration at initial pH 4.5. The adsorbent dose was varied from 200mg to 1g/L. It is observed that the removal of Pb (II) ions increases with an increase in the adsorbent dose. Removal of Pb (II) ions increases with increase of adsorbent dosage. The percentage removal increases from 30 to 70% by increasing the adsorbent dosage from 200mg to 1 g/L.
Adsorption Isotherms
Equilibrium isotherm equations are used to describe the experimental adsorption data. The parameters obtained from the different models provide important information on the sorption mechanisms and the surface properties and affinities of the adsorbent. The most widely accepted surface adsorption models for single-solute systems are the Langmuir and Freundlich models. The correlation with the amount of adsorption and the liquid-phase concentration was tested with the Langmuir and Freundlich isotherm equations. Linear regression is frequently used to determine the best-fitting isotherm, and the applicability of isotherm equations is compared by judging the correlation coefficients.
Freundlich Isotherm
The sorption data of nickel ions sorption onto AETB was also fitted to Freundlich isotherm, in the following linear form
log qe = log Kf + 1/n log Ce ....(1)
Where, qe is the amount of metal ion adsorbed per gram of adsorbent (mg/g). Ce is the equilibrium concentration of metal ion in solution (mg/L). Kf and 1/n are Freundlich constants, indicating the adsorption capacity and adsorption intensity, respectively.
Straight lines were obtained by plotting log qe against log Ce, which show that sorption of nickel ions obeys Freundlich isotherm well. The Kf and 1/n values were calculated from intercept and slop of the plot respectively and presented in Table 1. The correlation coefficient R2> 0.923 and the values of n were higher than 1.0, indicating that adsorption of Pb (II) ions on AETB follows the Freundlich isotherm.
Langmuir Isotherm
The Langmuir isotherm is valid for sorption of a solute from a liquid solution as monolayer adsorption on a surface containing a finite number of identical sites. Langmuir isotherm model assumes uniform energies of adsorption onto the surface without transmigration of adsorbate in the plane of the surface. The Langmuir isotherm is represented in the linear form as:
Ce / qe = 1/ b Q0 + Ce / Q0
Q0 and b is Langmuir constants related to the capacity and energy of sorption respectively. A plot of Ce/ qe versus Ce should indicate a straight line of slope 1/ Q0 and an intercept of 1/ (b Q0). The values of Qo and b and correlation coefficient obtained from the Langmuir model are shown in Table 1. The correlation coefficient R2 > 0.944 suggests that adsorption of Pb (II) ions onto AETB follows the Langmuir isotherm. The maximum monolayer capacity Q0 obtained from the Langmuir is 22.72 mg/g.
Conclusion
Adsorption of Pb (II) ions, from aqueous solutions using AETB studied. The following results were obtained:
- These studies show that Ailanthus Excelsa tree bark is an inexpensive adsorbent for Pb (II) removal from aqueous solutions.
- The adsorption of Pb (II) ions on AETB was dependent on the pH, initial Pb (II) ions concentration, quantity adsorbent dose and contact time.
- pH 4.5 was used as the optimum pH.
- The equilibrium time for the adsorption of Pb (II) ions on AETB from aqueous solutions is estimated 360 minutes.
- The adsorption process of Pb (II) ions can be described by Langmuir isotherm and Freundlich isotherm model
- The amount of Pb (II) ions adsorbed increased with increase initial Pb(II) ions concentration.
- Kinetic of Pb (II) ions adsorption obeyed the pseudo-second-order model.
References
- Davydova S., Heavy metals as toxicants in big cities, Microchemical Journal, 79, 133–136, (2005)
- Brinza L., Nygård C.A., Dring M.J., Gavrilescu M., & Benning L.G., Cadmium tolerance and adsorption by the marine brown alga Fucus vesiculosus from the Irish Sea and the Bothnian Sea, Bioresource Technology,100, 1727–1733,(2009)
- Karthika C., Vinnilamani N., Pattabhi S., & Sekar M.,Utilization of Sago Waste as an Adsorbent.(2010)
- Removal of Pb(II) from Aqueous Solution: Kinetics and Isotherm Studies, International Journal of Engineering Science and Technology, Vol. 2 (6), 1867–1879.
- Sannasi P. and Salmija S., Oriental Journal of Chemistry, Vol. 27, No. 2, 461-467, (2011)
- Zavvar Mousavi H., Seyedi S.R., Int. J. Enviro. Sci. Tech., 8(1), 195-202, (2011).
- Kafia M., Shareef Surchi, Agricultural Wastes as Low Cost Adsorbents for Pb Removal: Kinetics, Equilibrium and Thermodynamic,International Journal of Chemistry ,Vol. 3, No. 3; (2011)
- Kuchekar R Shashikant , Gaikwad B Vishwas , Sonawane V Dadasaheb and Lawande P Shamarao , Adsorption of Pb (II) ions on Tamarindous indica seeds as a low cost abundantly available natural adsorbent , Pelagia Research Library, 2(6),281-287, (2011)






