A review of Dichlorvos toxicity in fish
SUCHISMITA DAS1 *
DOI: http://dx.doi.org/10.12944/CWE.8.1.08
Copy the following to cite this article:
Das S. A review of Dichlorvos toxicity in fish. Curr World Environ 2013;8(1) DOI:http://dx.doi.org/10.12944/CWE.8.1.08
Copy the following to cite this URL:
Das S. A review of Dichlorvos toxicity in fish. Curr World Environ 2013;8(1). Available from: http://www.cwejournal.org/?p=3061
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2013-02-08 |
|---|---|
| Accepted: | 2013-02-23 |
Use of pesticide has become a necessary evil for developing countries like India where it is estimated that approximately 30% of its crop yield valued at Rs.60,000 crores are lost due to pest attack each year.1 Amongst others, organophosphorus pesticides (OPs) are the most commonly used pesticides in the world due to their quick degradation.2 Unfortunately, OPs lack target specificity and can cause severe, long lasting population effects on terrestrial and aquatic non-target species, particularly vertebrates.3 Quick degradation is probably the reason why, irrespective of reports of health hazardous, developing countries especially, in the Asia-Pacific region, use these chemicals for agricultural and public health purposes.4,5 Dichlorvos (2, 2-dichlorovinyl dimethyl phosphate) was first introduced in 1961.6
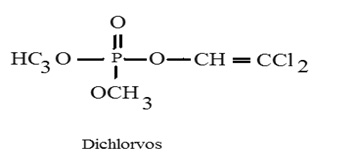
It has a molecular formula C4H7Cl2O4P and molecular weight to be 220.98 (Fig 1). It is also known by its trade name DDVP, Dedevap, Nogos,Nuvan, phosvit or Vapona.7 It is one of the most commonly used organophosphate pesticides in developing countries.8 It is classified by the WHO as a Class IB, 'highly hazardous'chemical.9 Dichlorvos is usually used as an agricultural insecticide on crops and stored products but is also used as an anti-helminthic (worming agent) for dogs, swine, and horses, as a botacide; agent that kills fly larvae.10 It is poisonous if swallowed, inhaled, or absorbed through the skin.11 It is extremely toxic pesticides to aquatic organisms and hampers fish health through impairment of metabolism sometimes leading to death. As one of the few organophosphates still registered for use, dichlorvos has elicited worldwide concern for many reasons. Although dichlorvos serves as a contact and stomach insecticide for food and non-food crop pests, it is also toxic to fish and other aquatic organisms.12-14 Dichlorvos is also commonly used in fish farming to eradicate crustacean ectoparasites.
It is specially used in the treatment of sea lice (Lepophtheirus salmonis and Caligus elogatus) on commercial salmon farms. But this pesticide often ends up producing both lethal and sub-lethal effects on the fish15 and even zooplanktons.16 At only 1 ppm, dichlorvos, showed both acute and chronic toxicity in fish.17 Some other workers have also noted adverse effects of dichlorvos in fish.18-20 The present study is an attempt to review the potential adverse effects of dichlorvos in fish. Fig. 1 Dichlorvos (2, 2-dichlorovinyl dimethyl phosphate)
ACUTE TOXICITY OF DICHLORVOS ON FISH The acute toxicity of dichlorvos to fish has been previously determined by a number of researchers. Its toxicity for freshwater and estuarine fish is moderate to high, and it does not bioaccumulate in fish.21 For freshwater and estuarine fish, 96h-LC50 values range from 0.2 to 12 mg/L.22 For marine fish, the toxicity was estimated to be more than 4 mg/L for adults and pre-adults of Atlantic salmon (Salmo salar).23 The 96h-LC50 value of dichlorvos obtained for fingerlings of European sea bass (Dicentrarchus labrax) was 3.5 mg/L.15 A comparison of the 96h-LC50 values published for several teleost fish species22 indicates that fingerlings of the European sea bass are more resistant to dichlorvos exposure than the most part of the other species of estuarine and freshwater fish studied.
However, the comparison with fathead minnow (Pimephales promelas) or with mosquito fish (Gambusia affinis) of similar size indicates that sea bass fingerlings are more sensitive to dichlorvos, since 96h-LC50-values of 12 and 5.3 mg/L have been reported22 for both species, respectively. In a study, it was found that 100% of 100 g salmon (Salmon salar) survived after 24 h of exposure to 1,3, and 5 mg/L of dichlorvos.24 The 96h-LC50 value of dichlorvos obtained for Labeo rohita was 16.71ppm. The fish in the same study exhibited erratic swimming, copious mucus secretion, loss of equilibrium and hitting to the walls of test tank prior to mortality in acute toxicity tests.25 The 96-h LC50values of Dichlorvos has been reported in Cirrhinus mrigala to be 9.1ppm,26 in Zebra fish, the 24-hpost fertilization LC50 value of dichlorvos in the semi static test was 39.75 mg/L27 and48-h LC50 values to be 0.5-10mg/L of dichlorvos formulations in carp.28 A study report indicated that 96-h LC50 value in rainbow trout was 0.93 mg/L29 and on golden orfe was 0.45mg/L.30 In another study, 100% lethality at 10 mg/L in fry of rainbow trout was found.31 In Tilapia mossambica with three size groups, 96-h LC50 values were found to be 1.4-1.9mg/L, the smaller sizes being more sensitive.32 24, 48 and 96-h LC50 values of dichlorvos in common carp to be 3.8, 2.7, 2.3mg/L respectively and 4.1, 4.0 and 3.7 mg/L in Java carp respectively.33 In harlequin fish found 24-h LC50 value to be 12mg/L and 48-h LC50 value to be 7.8mg/L34 while 24 and 48-h LC50 in bluegill sunfish to be 1 and 0.7mg/L respectively.35 Again, 96-h LC50 in bluegill and spots were reported to be 0.48 and 0.55mg/L respectively.36 CHRONIC TOXICITY OF DICHLORVOS ON FISH
Effects on Choline Esterase Activity
Dichlorvos is an organophosphorus insecticide reported to be neurotoxic due to its irreversible inhibitory effect on AChE.37 The enzyme AChE degrades the neurotransmitter acetylcholine in cholinergic synapses. The inhibition provokes an accumulation of acetylcholine in synapses with disruption of the nerve function that can end in the death of the organism. Several authors have been reporting significant inhibition of ChE activity in fish at sub-lethal concentrations of dichlorvos.38-41 In sea bass, dichlorvos significantly inhibited the activity of ChE in the selected tissues, both in vitro and in vivo conditions. Differences in ChE sensitivity were found in relation to the age of the fish and the tissue analysed. Sea bass fingerlings are able to tolerate high levels of head and muscle ChE inhibition before death.15 Similar results were obtained for pinfish (Lagodon rhomboides)42 and European eel (Anguilla anguilla).43
Brain tissue showed higher ChE activity than muscle, whole blood or plasma tissues. On the contrary, in fingerlings, the highest ChE activity was obtained in muscle. This is in agreement with the results obtained by other researchers.44-46 Skeletal muscle presented higher ChE activity than brain tissue in juveniles of goldfish (Carassius auratus) of size ~5 g exposed to three different pesticides.47 Chronic dichlorvos exposure impaired mitochondrial energy metabolism and neuronal apoptotic cell death in brain.48 AChE activity of Tilapia mossambica in relation to the interacting effects of aging and sub-lethal concentrations of dichlorvos was studied.49 The enzyme activity of brain and liver decreased with increasing size (and age) and dichlorvos exposed fish showed considerable inhibition of brain and liver AChE. There was a positive correlation between dichlorvos concentration and the time of exposure when the degree of enzyme inhibition was considered. Brain exhibited a higher degree of enzyme inhibition in all age groups of fish as compared to liver. Small fish were more susceptible to the insecticide with respect to AChE activity. When transferred to clean water, most of the exposed fishes recovered their AChE activity and the recovery was greater in liver than in brain.49
Effects on Antioxidants
It has now been established that OP pesticides induced oxidative stress.50 An antioxidant defence system (ADS) is needed to protect biomolecules from the harmful effects of ROS. Fish are endowed with defensive mechanisms to neutralize the impact of reactive oxygen species (ROS) resulting from the metabolism of various chemicals. These include various antioxidant defence enzymes such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPOx), glutathione S-transferase (GST), and glutathione reductase (GR). Low molecular weight antioxidants such as glutathione (GSH), ascorbate (vitamin C), vitamin A and E are also reported to contribute in the quenching of oxy radicals.51
ROS which is not neutralized by this antioxidant defense system damages all biomolecules. One of the most important targets of ROS is the membrane lipids which undergo peroxidation (LPO). Thus, LPO estimation has also been successfully employed to signify oxidative stress induced in aquatic animals by such chemicals.52 Decreased GSH levels and also decreased MnSOD activity were observed, in the brain mitochondria isolated from low-level chronic dichlorvos treated rat.43 Also, in fish exposed to dichlorvos for 24 h, at concentrations of 1 or 5 mg/L, a dose-dependent increase was noted in the activities of SOD and CAT in the liver and brain. A rise was also observed in the level of GSH and changes were noted in MDA level in these organs. The increase in GSH was noted mainly in the brain and was accompanied by a decrease in MDA level. The decrease was greater at the exposure to the higher dose of the compound.53
Chromosomal Aberrations and Carcinogenic Effects
Dichlorvos concentration of 0.01 ppm caused chromosomal aberrations in the form of centromeric gaps, chromatid gaps, chromatid breaks, sub-chromatid breaks, attenuation, extra fragments, pycnosis, stubbed arms etc in kidney cells of Channa punctatus after exposure periods of 24, 48, 72 and 96 h.54 Interestingly, there was an inverse relationship between duration of exposure and aberration frequency. Longer exposures to dichlorvos were associated with lower frequencies of aberrations. The toxicity of dichlorvos has also been related to alterations in DNA replication, which causes mutations55 and cellular hyperproliferation as a result of local irritation.56-58 Dichlorvos has carcinogenic potential, which has been reviewed on several earlier occasions by several workers.59-62
Immune Response
Dichlorvos has the potential to induced altered immune response in fish and it was reviewd by Dunier et al., 1991.63
Developmental Effects
Dichlorvos exposure during early development in Zebra fish caused clear behavioural impairments detectable during the post hatching period. It also showed mortality and developmental abnormalities.27
Histopathology
Histopathology is an important tool in assessing pesticide toxicity.64 The histopathological effects of liver tissues in Cirrhinus mrigala chronically exposed to dichlorvos showed hepatic lesions in the liver tissues were observed which were characterized by cloudy swelling of hepatocytes, congestion, vacuolar degeneration, karyolysis, karyohexis, dilation of sinusoids and nuclear hypertrophy. In the same study, changes in gills such as hyperplasia, desquamation, and necrosis of epithelial, epithelial lifting, oedema, lamellar fusion, collapsed secondary lamellae, curling of secondary lamellae and aneurism in the secondary lamellae were observed after exposure to dichlorvos.26 The effects of sub-lethal doses of dichlorvos on lipid composition and metabolism of rainbow trout skin cells in primary culture were investigated and it was suggested that dichlorvos may have direct effects on fish skin that could have important consequences for fish health in general.65 In another study in air-breathing catfish Clarias batrachus exposed to lethal and sublethal concentrations of the dichlorvos, significant cytoarchitectural changes in the oocytes, including pronounced vacuolation, degeneration and deformation were observed.66
Clumping of the cytoplasm and karyohypertrophy were also evident and rupture of the cell wall, with extrusion of the cytoplasm and the nuclei, was observed in the same study.66 Pesticides are also reported to cause changes in structure and functions of fish gonads.67 Although the studies with dichlorvos are scarce, the effects of sublethal concentrations of dichlorvos (0.65 mg/l, 0.90 mg/l and 1.17 mg/l) on the gonadosomatic index of the fish, Cyprinus carpio communis was studied.68 The Ganadosomatic index decreased with the increase in concentration, whereas it increased with increase in exposure at all concentrations.68
Conclusion
Dichlorvos toxicity in fish has been studied by several workers who have shown that at chronic level, it causes diverse effects including oxidative damage, inhibition of AchE activity, histopathological changes as well as developmental changes, mutagenesis and carcinogecity. With reports of dichlorvos usage and its adverse effects on non-target organisms like fish, it has become essential to formulate stringent rules against indiscriminate use of this pesticide. Since dichlorvos is present in the environment with other similar organophosphate compounds, additive responses to organophosphate compounds may induce lethal or sublethal effects in fish. It is, therefore, a matter of great public health significance to regularly monitor the pesticide residues in foods and humans in order to assess the population exposure to this pesticide. Besides, for a safe use of this insecticide more experimental work should be performed to determine the concentration and time of exposure that do not induce significant sub-lethal effects on fish.
References
- http://www.nabard.org/modelbankprojects/land_development.asp retrieved on 11 January, 2013.
- Eto, M. (1974) Organophosphorus Pesticides: Organic and Biological Chemistry, CRC Press.
- Rahimi, R. Nikfar, S. and Abdollahi, M. (2006) Increased morbidity and mortality in acute human organophosphate-poisoned patients treated by oximes: a metaanalysis of clinical trials. Hum. Exp. Toxicol. 25: 157–162, http://dx.doi.org/10.1191/0960327106ht602oa
- Dave, P.P. (1996) India: A generic giant. Farm Chem Int., 10, 36–37.
- Li, Y. Mcmillan, A. and Scholtz, M.T. (1996) Global HCH usage with 1° _1° longitude/latitude resolution. Environ. Sci. Technol., 30: 3525–3533, http://dx.doi.org/10.1021/es960312v
- Mennear, J.H. (1998) Dichlorvos: A Regulatory Conundrum. Regulatory Toxicology and Pathology 27: 265–272, http://dx.doi.org/10.1006/rtph.1998.1217
- http://en.wikipedia.org/wiki/Dichlorvos retrieved on 11 January, 2013.
- Binukumar, B.K. and Gill, K.D. (2010) Cellular and Molecular mechanisms of dichlorvos neurotoxicity: Cholinergic, noncholinergic, cell signaling, gene expression and therapeutic aspects. Indian journal of experimental biology. 48: 697-709.
- World Health Organisation (WHO) (1992) International Programme on Chemical Safety, WHO Recommended Classification of Pesticide by Hazard and Guidelines to Classification 1994-1995, UNEP/ILO/WHO.
- USEPA: United States Environmental Protection Agency (1994) Integrated Risk Information System (IRIS) on Dichlorvos. Environmental Criteria and Assessment Office, Office of Health and Environmental Assessment, Office of Research and Development, Cincinnati, OH.
- Musa, U. Hati, S.S. Mustapha, A. and Magaji, G. (2010). Dichlorvos oncentrations in locally formulated pesticide (Ota-piapia) utilized in northeastern Nigeria. Scientific Research and Essay. 5: 49-54.
- Suntio, L.R. Shiu, W.Y. Glotfelty, D. MacKay, D. and Seiber, J.N. (1988) Critical review of Henry’s Law constants for pesticides. Rev. Environ. Contam. Toxicol. 103: 1–59, http://dx.doi.org/10.1007/978-1-4612-3850-8_1
- Naqvi, S.M. and Vaishnavi, C. (1993) Mini review. Bioaccumulative potential and toxicity of dichlorvos insecticide to non-target animals. Comp. Biochem. Physiol. C 105: 347–361, http://dx.doi.org/10.1016/0742-8413(93)90071-R
- Toledo, M.C.F. and Jonsson, C.M. (1992) Bioaccumulation and elimination of endosulfan in zebra fish (Brachydanio rerio). Pesticide Sci. 36: 207–211, http://dx.doi.org/10.1002/ps.2780360306
- Varo, I. Navarro, J.C. Amat, F. and Guilhermino, L. (2003) Effect of dichlorvos on cholinesterase activity of the European sea bass (Dicentrarchus labrax). Pesticide Biochemistry and Physiology 75: 61–72, http://dx.doi.org/10.1016/S0048-3575(03)00019-1
- Gupta, A. K.. Verma, G.P. and Jain, K.L. (2008) Acute toxicity of organophosphate insecticide, dichlorvos in relation to selected water hardness for the freshwater zooplankters. J Env Biol. 29: 837-839.
- Roberts, R.J. and Sheperd, C.J. (1986) Handbook of trout and salmon diseases. FishingNew Book, 2nd edition.
- Horsberg, T.E. Hoey, T. and Nafstad, I. (1989) Organophosphate poisoning of Atlantic salmon in connection with treatment against salmon lice. Acta Vet. Scand. 30: 385-390.
- Rajeswari, K. Reddy, S.J. Reddy, D.C. and Ramamurthi, R. (1989) Effect of dichlorvos on certain blood parameters of the fish Clarias batrachus. Environ. Ecol. 7: 933-934.
- Demael, A. Dunier, M. and Siwicki, A.R. (1990) Some effects of dichlorvos on carp metabolism. Comp. Biochem. Physiol. 95C: 237-240.
- Howard, P.H. (1991) Pesticides, Lewis Publisher, Chelsea, MI.
- World Health Organisation (WHO) (1989) Environmental health criteria for dichlorvos. Series, No. 79. Geneva, Italy.
- Roth, M. (2000) The availability and use of chemotherapeutic sea lice control products, Contribut. Zool. 69:109-118.
- Sievers, G. Palacios, P. Inostroza, R.and D’olz, H. (1995) Evaluation of the toxicity of 8 insecticides in Salmo salar and the in vitro effects againts the isopode parasite, Ceratothoa gaudichaudii. Aquaculture 134: 9-16, http://dx.doi.org/10.1016/0044-8486(95)00026-X
- Bhat, B.A. Bhat, I.A. Vishwakarma, S. Verma, A. and Saxena, G. (2012) A Comparative Study on the Toxicity of a Synthetic Pesticide, Dichlorvos and a Neem based Pesticide, Neem-On to Labeo rohita (Hamilton). Current World Environment. 7: 157-161.
- Velmurugan, B. Selvanayagam, M. Cengiz, E.I. and Unlu, E. (2009) Histopathological changes in the gill and liver tissues of freshwater fish, Cirrhinus mrigala exposed to dichlorvos. Brazilian Archives of Biology and Technology 52: 1291-1296, http://dx.doi.org/10.1590/S1516-89132009000500029
- Sisman, T. (2010) Dichlorvos-induced developmental toxicity in Zebrafish. Toxicol Ind Health. 26: 567-573, http://dx.doi.org/10.1177/0748233710373089
- Nishiuchi, Y. (1981) Toxicity of pesticides to some aquatic organisms – I Toxicity of pesticides to some aquatic insects. English translation from Seitai Kagaku 4: 31-46.
- Bayer (1980). Toxicity to fish. Report No. FF-110. Crop Protection Application Technology, Chemical Product Development and Ecobiology, Bayer AG, Leverkusen, Germany. Bayer Australia Ltd Agricultural Division, Dichlorvos TGAC Submission to the Technical Committee on Agricultural Chemicals. Book X, Part 7: Environmental toxicology, pp 7:058-060. Unpublished Report.
- Bayer (1981). Toxicity to fish. Report No. FO-373. Crop Protection Application Technology, Chemical Product Development and Ecobiology, Bayer AG, Leverkusen, Germany. Bayer Australia Ltd Agricultural Division, 60 Dichlorvos TGAC Submission to the Technical Committee on Agricultural Chemicals. Book X, Part 7: Environmental toxicology, pp 7:065-068. Unpublished Report.
- Lewallen, L.L. and Wilder, W.H. (1962) Toxicity of certain organophosphorus and carbamate insecticides to rainbow trout. Mosquito News 22: 369-372.
- Rath, S. and Misra, B.N. (1981) Toxicological effects of dichlorvos (DDVP) on brain and liver acetylcholinesterase (AChE) activity of Tilapia mossambica, Peters. Toxicology. 19: 239-45, http://dx.doi.org/10.1016/0300-483X(81)90133-5
- Koesoemadinata, S. (1983) Lethal toxicity of 24 insecticide formulations commonly used for rice pest control in irrigated rice field to two Indonesian freshwater fish species, Cyprinus carpio and Puntius gonionotus. Badan Pelelitian dan Pengembangan Pertanian, Balai Penelitian Perikanan Darat (Research Institute for Inland Fisheries, Bogor, Indonesia).
- Alabaster, J.S. (1969) Survival of fish in 164 herbicides, insecticides, fungicides, wetting agents and miscellaneous substances. Intern. Pest Control 11: 25-35.
- Pimentel, D. (1971). Ecological effects of pesticides on non-target species. Executive Office of the President, Office of Science and Technology. US Government Printing Office, Washington DC, USA, Stock No. 4106-0029 (excerpt only).
- Kenaga, E.E. (1979) Acute and chronic toxicity of 75 pesticides to various animal species. Down to Earth 35: 25-31.
- Wang, H.H. Chou, Y.C. Liao, J.F. and Chen, C.F. (2004) The effect of insecticide dichlorvos on esterase activity extracted from the Psocids Liposceids bostrychophila and L. entomophilila. J. Insect Sci. 4: 1–5.
- Galgani, F. and Bocquene, G. (1990) In vitro inhibition of acetylcholinesterase from four marine species by organophosphates and carbamates. Bull. Environ. Contam. Toxicol. 45: 243-249, http://dx.doi.org/10.1007/BF01700191
- Bocquene, G. Bellanger, C. Cadiou, Y. and Galgani, F. (1995) Join action of combinations of pollutants on the acetylcholinesterase activity of several marine species. Ecotoxicology 4: 266-279, http://dx.doi.org/10.1007/BF00116345
- Sturm, A. Wogram, J. Hansen, P.D. and Liess, M. (1999) Potential use of cholinesterase in monitoring low levels of organophosphates in small streams: natural variability in three-spined stickleback (Gasterosteus aculeatus) and relation to pollution. Environ. Toxicol. Chem. 18: 194-200.
- Sturm, A. Wogram, J. Segner, H. and Liess, M. (2000) Different sensitivity to organophosphates of acetylcholinesterase and butyrylcholinesterase from threespined stickleback (Gasterosteus aculeatus): application in biomonitoring, Environ. Toxicol. Chem. 19: 1607-1615.
- Coppage, D.L. and Matthews, E. (1975) Head acetylcholinesterase inhibition in a marine teleost during lethal and sublethal exposures to 1-dichloroethyl dimethyl phosphate (Naled) in seawater. Toxicol. Appl. Pharmacol. 31: 128-133, http://dx.doi.org/10.1016/0041-008X(75)90060-5
- Sancho, E. Ferrando, M.D. and Andreu-Moliner, E. (1997). Response and recovery of head acetylcholinesterase activity in the European eel, Anguilla Anguilla. Ecotoxicol. Environ. Safe. 38: 205-209, http://dx.doi.org/10.1006/eesa.1997.1579
- Ceron, J.J. Ferrando, M.D. Sancho, E. Gutierrez- Panizo, C. and Andreu-Moliner, E. (1996) Effects of diazinon exposure on cholinesterase activity in different tissues of european eel (Anguilla anguilla). Ecotoxicol. Environ. Safe. 35: 222-.225.
- Straus, D.L. and Chambers, J.E. (1995) Inhibition of acetylcholinesterase and aliesterases of fingerling channel catfish by chlorpyrifos, parathion, and s,s,s-tributyl phosphorotrithioate (DEF). Aquat. Toxicol. 33: 311-324, http://dx.doi.org/10.1016/0166-445X(95)00024-X
- Sancho, E. Ceron, J.J. and Ferrando, M.D. (2000). Cholinesterase activity and hematological parameters as biomarkers of sublethal molinate exposure in Anguilla anguilla, Ecotoxicol. Environ. Safe. 46: 81-86, http://dx.doi.org/10.1006/eesa.1999.1888
- Bretaud, S. Toutant, J.P. and Saglio, P. (2000) Effects of carbofuran, diuron, and nicosulfuron on acetylcholinesterase activity in goldfish (Carassius auratus). Ecotoxicol. Environ. Safe. 47, 117-124, http://dx.doi.org/10.1006/eesa.2000.1954
- Kaur, P. Radotra, B. Minz, R.W. and Gill, K.D. (2007) Impaired mitochondrial energy metabolism and neuronal apoptotic cell death after chronic dichlorvos (OP) exposure in rat brain. Neurotoxicology 28, 1208–1219, http://dx.doi.org/10.1016/j.neuro.2007.08.001
- Rath, S. and Misra, B.N. (1979). Relative toxicity of dichlorvos (DDVP) to Tilapia mossambica Peters of 3 different age groups. Exp. Gerontol. 14: 307-309, http://dx.doi.org/10.1016/0531-5565(79)90042-1
- Lukaszewicz-Hussain, A. (2010) Role of oxidative stress in organophosphate insecticide toxicity – Short review. Pesticide Biochemistry and Physiology 98: 145–150, http://dx.doi.org/10.1016/j.pestbp.2010.07.006
- Kalender, S. Ogutcu, A. Uzunhisarcikli, M. Acikgoz, F. Durak, D. Ulusoy, Y. and Kalender, Y. (2005) Diazinon-induced hepatotoxicity and protective effect of vitamin E on some biochemical indices and ultrastructural changes. Toxicology 211, 197–206, http://dx.doi.org/10.1016/j.tox.2005.03.007
- Lukaszewicz-Hussain, A. and Moniuszko-Jakoniuk, J. (2004) Chlorfenvinphos, an organophosphate insecticide, affects liver mitochondria antioxidative enzymes, glutathione and hydrogen peroxide concentration. Pol. J. Environ. Stud. 13: 397–401.
- Hai, D.Q. Varga, S. I. and Matkovics, B. (1997) Organophosphate effects on antioxidant system of Carp (Cyprinus carpio) and Catfish (Ictalurus nebulosus). Comp. Biochem. Pharmacol. 117C, 83–88.
- Rishi, K.K. and Grewal, S. (1995) Chromosomal aberration test for the insecticide, dichlorvos, on fish chromosomes. Mutation research, 344: 1-4, http://dx.doi.org/10.1016/0165-1218(95)90032-2
- Gilot-Delhalle, J. Colizzi, A. Moutshen, J. and Moutshen-Dahmen, M. (1983) Mutagenicity of some organophosphorus compounds at the ade6 locus of Schizosaccharomyces pombe. Mutat. Res. 117: 139-148, http://dx.doi.org/10.1016/0165-1218(83)90161-1
- Mirsalis, J.C. Tyson, C.K.. Steinmetz, K.L. Loh, E.K.. Hamilton, C.M. Bakke, J.P. and Spalding, J.W. (1989) Measurement of unscheduled DNA synthesis and S phase synthesis in rodent hepatocytes following in vivo treatment: testing of 24 compounds. Environ. Mol. Mutagen. 14: 155-164, http://dx.doi.org/10.1002/em.2850140305
- Oshiro, Y. Piper, C.E. Balwierz, P.S. and Soelter, S.G. (1991) Chinese hamster ovary cell assays for mutation and chromosome damage: data from non-carcinogens. J. Appl. Toxicol. 11: 167-177, http://dx.doi.org/10.1002/jat.2550110304
- Benford, D.J. Price, S.C. Lawrence, J.N. Grasso, P. and Bremmer J.N. (1994). Investigations of the genotoxicity and cell proliferative activity of dichlorvos in mouse forestomach. Toxicology 92: 203-215, http://dx.doi.org/10.1016/0300-483X(94)90178-3
- Bremmer, J.N. Walker, A.I.T. and Grasso, P. (1988) Is dichlorvos a carcinogenic risk for human? Mutation research 209, 39-44, http://dx.doi.org/10.1016/0165-7992(88)90108-X
- Mennear, J. H. (1994) Dichlorvos carcinogenicity: An assessment of the weight of experimental evidence. Regul. Toxicol. Pharmacol. 20: 354–361, http://dx.doi.org/10.1006/rtph.1994.1080
- International Agency for Research on Cancer (IARC) (1987). Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs, Volumes 1 to 42. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Supplement 7.
- National Toxicology Program (NTP) (1989). Toxicology and carcinogenesis studies of dichlorvos in F344/N rats and B6C3F1 mice. U.S. Dept. of HHS, PHS, NIH, NTP Technical Report No. 334.
- Dunier, M. Siwicki, A.K. and Demael, A. (1991) Effects of organophosphorus insecticides: effects of trichlorfon and dichlorvos on the immune response of carp (Cyprinus carpio).III. In vitro effects on lymphocyte proliferation and phagocytosis and in vivo effects on humoral response. Ecotoxicol. Environ. Safety 22: 79-87, http://dx.doi.org/10.1016/0147-6513(91)90049-U
- Das, S. and Gupta, A. (2012) Effect of Malathion (EC50) on Gill Morphology of Indian Flying Barb, Esomus danricus (Hamilton-Buchanan). World Journal of Fish and Marine Sciences 4: 626-628.
- Ghioni, C. Tocher, D. R. and Sargent, J. R. (1998) Effects of dichlorvos and formalin on fatty acid metabolism of rainbow trout (Oncorhynchus mykiss) skin cells in primary culture. Fish Physiol biochem, 18: 241-252, http://dx.doi.org/10.1023/A:1007730730655
- Benarji, G., and Rajebdranath, T. (1991) Dichlorvos-induced histoarchitectural changes in the oocytes of a freshwater fish. Funct Dev. Morphol. 1: 9-12.
- Singh, H. and T.P. Singh, 1982. Effect of pesticides on fish reproduction. Ichthyologia, 15: 71-81.
- Mir, F.A. Shah, G.M. Jan, U. and Mir, J.I. (2012) Studies on Influences of Sublethal Concentrations of Organophosphate Pesticide; Dichlorvos (DDVP) on Gonadosomatic Index (GSI) of Female Common Carp, Cyprinus carpio communis. American-Eurasian Journal of Toxicological Sciences 4: 67-71.






