Pyridine-Functionalized TiO2 Nanoparticles as a Sorbent for Preconcentration and Determination of Ultra-Trace Palladium Ion
Mohammad Karimi1 * , Mona Feiz Bakhsh Bazargani2 , Forouzan Aboufazeli1 , Hamid Reza Lotfi Zadeh Zhad1 , Omid Sadeghi1 and Ezzatollah Najaf *
DOI: http://dx.doi.org/10.12944/CWE.7.2.06
Copy the following to cite this article:
Karimi M, Bazargani M.F.B, Aboufazeli F, Zhad H.R.L.Z, Sadeghi O, Najafi E. Pyridine-Functionalized TiO2 Nanoparticles as a Sorbent for Preconcentration and Determination of Ultra-Trace Palladium Ions. Curr World Environ 2012;7(2):227-232 DOI:http://dx.doi.org/10.12944/CWE.7.2.06
Copy the following to cite this URL:
Karimi M, Bazargani M.F.B, Aboufazeli F, Zhad H.R.L.Z, Sadeghi O, Najafi E. Pyridine-Functionalized TiO2 Nanoparticles as a Sorbent for Preconcentration and Determination of Ultra-Trace Palladium Ions. Curr World Environ 2012;7(2):227-232. Available from: http://www.cwejournal.org?p=380/
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2012-07-12 |
|---|---|
| Accepted: | 2012-09-17 |
Introduction
Increasing demand for noble metals, particularly platinum group metals (PGM), such as palladium(II), platinum(IV), ruthenium(III), rhodium(III) has been recently observed because of their wide range of industrial applications, e.g. as catalysts in organic processes, value added components in metal alloys and vehicle catalytic converter systems, in chemical, pharmaceutical, petroleum and electronic industries and also in jewellery making.
These applications of PGMs have increased the demand for these metals, whereas the natural resources are limited.1,2
Flame atomic absorption spectrometry (FAAS) is one of the most popular techniques for determination of metal ions because of its high specificity and low cost. However its sensitivity is usually insufficient for determination of trace metal ions in environmental samples. In order to overcome this problem and prevent interference effects, those who use this method usually include an efficient preconcentration step.3,4
Solid-phase extraction (SPE) is one of the most common methods for preconcentration of noble metals. It has the advantages of flexibility, economical and environmental-friendly, simplicity, and being fast and safe.5 Since the key point in SPE is choosing adsorbent, several SPE methods based on sorbents such as different polymers,6 silica 7 and Fe3O4 8 have been developed. Compared to the other sorbents TiO2 has attracted more attention due to its high surface area.9
In this work, a novel sorbent based on functionalization of TiO2 nano-particles by pyridine group is constructed. This sorbent was applied for preconcentration of Pd(II) ions in aqueous samples after characterization by FT-IR, XRD pattern, elemental analysis and SEM micrograph.
Experimental and Methods
Reagents and Materials
The standard solution of Pd(II), 1000 mg L-1, was purchased from Aldrich Company (Milwaukee, Wi, USA). TiO2 nano-particles with 10-15 nm in diameter were purchased from Neunano Company (Tehran, Iran). All reagents includes solvents, acids, 3-aminopropyltriethoxysilane, triethylamine, dichloromethane, oxalyl chloride and 4-pyridine carboxylic acid were of analytical grade and purchased from Merck Company (Darmstadt, Germany). The standard reference material (NIST SRM 2557) was purchased from National Institute of Standards.
Apparatus
The atomic absorption spectrometer (AAS) used in this experiment was a Shimadzu AA-680 equipped with a single element hollow cathode lamp (6.0 mA for palladium). Burner head was 50 mm and air acetylene burner head was used during the experiment. Resonance line for palladium is 244.8 nm, so the wavelength was set at this value. The spectral band width was set at 0.5 nm and the ratio of air-acetylene was set at 4.7. The pH was measured at 25 ±1 ºC with a digital WTW Metrohm 827 Ion analyzer (Herisau, Switzerland) equipped with a combined glass-calomel electrode. The Fourier Transform Infrared (FT-IR) spectrum was recorded on a BOMEM MB-Series FT-IR spectrometer in the form of KBr pellets. The elemental analyses (CHNS) were performed on a Thermo Finnigan Flash-2000 microanalyzer (Italy). The SEM micrograph was recorded by a Vega-TeScan scanning electron microscope.
Preparation of Pyridine functionalizing agent
Pyridine functionalizing agent was synthesized according to earlier report10 and characterized by 1H NMR. Briefly, 1.0 g of 4-pyridine carboxylic acid was suspended in 100 mL of dried CH2Cl2 under nitrogen atmosphere and 10 mL of oxalyl chloride was slowly added to the mixture and was stirred for 12 h. Then CH2Cl2 was removed under reduced pressure, and the residue was suspended again in 100 mL of dried CH2Cl2. After addition of 17 mL triethylamine to reaction mixture, 4.0 g 3-aminopropyltrimethoxysilane was slowly added. The reaction mixture was stirred at room temperature for further 4 h. Then the solvent was removed under reduced pressure to obtain brownish viscose oil.
Preparation of Pyridine Functionalized TiO2 Nano-Particles
In a typical reaction, 1.0 g TiO2 nano-particles were suspended in 50 mL toluene, and 2 mL pyridine functionalization agent was added and the mixture was refluxed for 24 h under nitrogen atmosphere. Then the solid was collected by filtration and washed with methanol and acetone and then dried at room temperature. Formation of pyridine functionalized TiO2 nano-particles (Py-TiO2 NPs) was confirmed by FT-IR spectroscopy, XRD pattern, elemental analyses and SEM micrograph.
Column Preparation
A glass column, 120 mm in length and 20 mm in diameter, was blocked by polypropylene filters at the ends, filled with 200 mg of the Py-TiO2 nano-particles, and then used for the experiments. Before extraction, the column was treated with 5 mL hydrochloric acid (1 M), 5 mL nitric acid (1 M), 5 mL toluene, 5 mL ethanol and 20 mL distilled water to remove organic and inorganic contaminants.
Preconcentration Procedure
A solution containing 1 µg mL-1 of palladium with pH=7.0 was prepared. The pH was adjusted with Na2HPO4/ NaH2PO4 buffer solution and then 50 mL of solution was passed through the column at a flow rate of 8 mL min−1. The column was eluted by 12 mL of 1 mol L−1 thiourea in 0.1 mol L−1 HCl solution, then the eluent was analyzed by FAAS.
Standard Reference Materials Ppretreatment
Auto-catalyst NIST SRM 2557 of 0.1000 g were mixed with about 5.0 mL aqua regia and 1.0 mL HF (48–51%, v/v) in a Teflon vessel, and heated until the sample was completely decomposed. Then the solutions were evaporated in a water bath. The residues were dissolved with 0.05 mol l−1 HCl and diluted to the appropriate volume with distilled water.11
Results and Discussion
Sorbent Characterization
Modification of TiO2 nano-particles have been performed according to previous report.12 Reaction of pyridine functionalizing agent with active hydroxyl group on the surface of TiO2 leads to formation of this sorbent (Fig. 1). Formation of this sorbent was confirmed by FT-IR spectroscopy, XRD pattern, elemental analyses and SEM micrograph. The presence of peaks at 3027 (CH, aromatic), 2953 (CH, aliphatic), 1561&1470 (C=C, aromatic) and 1402 (C=N) in IR spectrum confirm presence of pyridine in this sorbent. Also the amount of grafted pyridine was calculated by elemental analysis. According to the elemental analysis results (%C= 6.74, %H= 0.69, %N= 1.73), approximately 0.61 mmol pyridine is grafted on each gram of TiO2 nano-particles. In order to confirm remaining TiO2 nano-particles unchanged after functionalization (no decomposition or converting to the other oxides), XRD pattern of final product was recorded. Comparing to reference pattern (JCPDS file, No. 86–0147), the results show the TiO2 nanoparticles structure has not been changed after functionalization (Fig. 2). Finally in order to investigate the size and morphology of this sorbent, SEM micrograph of Py-TiO2 nano-particles was recorded. As it can be seen in Fig. 3, spherical nanoparticles with approximately 15-20 nm in diameter were obtained.
Optimization Studies
Influence of pH In order to study the effect of pH on the Pd(II) extraction, the pH of 50 mL of different sample solutions containing 1 mg L−1 palladium were adjusted in the range of 2-9. The samples were passed through the column at a flow rate of 8 mL min−1. Then the column was eluted by 12 mL of 1 mol L−1 thiourea in 0.1 mol L−1 HCl solution and the Pd(II) content in eluent was analyzed by FAAS. As the results in Fig. 4 show, the highest palladium recovery is at pH=7.0. The best recovery at neutral pH may be attributed to the presence of free lone pair of electrons on the nitrogen atoms which are suitable donors for coordination to the palladium ions. Effect of type, concentration and volume of eluent Different eluent solutions including different HCl solutions and their mixture with thiourea with different concentrations were used for desorption of palladium from Py-TiO2 nano-particles. In this approach, a solution containing 1 µg mL-1 of palladium with pH=7.0 was passed through the column at a flow rate of 8 mL min−1. Then the adsorbed ions were desorbed by 20 mL of each eluent. Then the palladium content in each eluent was analyzed by FAAS. According to these results the best eluent is a solution of 1 mol L−1 thiourea in 0.1 mol L−1 HCl. Moreover in order to study the effect of elunet volume, different volume of 1 mol L−1 thiourea in 0.1 mol L−1 HCl solution (2 , 4, 6, 8, 10, 12, 14, 16 and 18 mL) was used for palladium desorption. The results show that at least 12 mL of this eluent is needed for complete palladium desorption from the column.
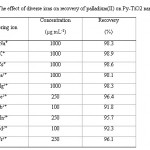 |
Table 1: The effect of diverse ions on recovery of palladium(II) on Py-TiO2 nano-particles Click here to View table |
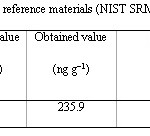 |
Table 2: The analysis of standard reference materials (NIST SRM 2557) Click here to View table |
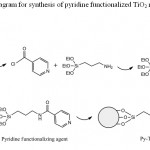 |
Figure 1: A schematic diagram for synthesis of pyridine functionalized TiO2 nanoparticles Click here to View figure |
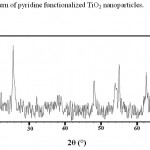 |
Figure 2: The XRD pattern of pyridine functionalized TiO2 nanoparticles Click here to View figure |
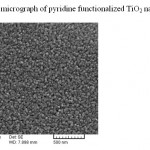 |
Figure 3: SEM micrograph of pyridine functionalized TiO2 nanoparticles Click here to View figure |
Sample and eluent flow rates In order to study sample and eluent flow rates, the pH of 100 mL of 1 µg mL-1 palladium (II) solution was adjusted to 7 and the solution was passed through the column with different flow rates in the range of 1-10 mL min-1 using a peristaltic pump. As Fig. 5 show, the maximum flow rate for complete adsorption is 8 mL min-1. Also same experiments were performed by different eluent flow rates. As it is shown in Fig. 5, at the flow rates more than 2 mL min-1, the Pd(II) desorption will be decrease. So in the further experiments, 8 and 2 mL min-1 were choosed as optimum sample and eluent flow rates, respectively.
Influence of Interference Ions
To investigate the selectivity of the sorbent, the effect of different cations such as Na+, K+, Cs+, Mg2+, Ca2+, Fe2+, Pb2+, Mn2+, Cd2+ and Cr3+ in the Pd(II) determination was studied. The cations of as their chloride salts with various concentrations were added to a 100 mL of single solution containing 1 µg mL-1 palladium (II) and the extraction procedure was followed. As can be seen from Table 1, a good selectivity for palladium extraction was observed in pH=7.0 and this sorbent could be used as a selective Pd(II) extractor in natural samples with diverse interfere ions.
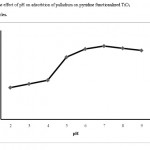 |
Figure 4: The effect of pH on adsorbtion of palladium on pyridine functionalized TiO2 nanoparticles Click here to View table |
 |
Figure 5: Investigation of adsorption and desorption time of palladium on pyridine functionalized TiO2 nanoparticles Click here to View table |
Maximum Adsorption Capacity
In order to determine the maximum adsorption capacity of this sorbent, 500 mL of a solution containing 100 mg palladium was treated with the extraction procedure and the maximum capacity was calculated by analyzing the adsorbed palladium in eluent. The maximum adsorption capacity for three replicates was found to be 61 mg g-1 (0.57 mmol g-1).
Analytical Performance
In order to determine the detection limit (DL) of the presented method, 500 mL of ten blank solutions were passed through the column under the optimal conditions. The LOD values of 3.8 ng mL−1 was obtained for palladium with Py-TiO2 nano-particles from CLOD= KbSb/m using a numerical factor of kb=3.The analytical values of the proposed method were calculated from the data obtained under the optimum conditions. The recovery of the extraction of palladium ion on Py-TiO2 was determined to be 99.1 % with a relative standard deviation of 2.5 % for ten replicated analysis.
Method Validation
The method validation was done by analyzing a standard reference material. NIST SRM 2557 was analyzed by this method and the results of this study are presented in Table 2. The obtained results were in a good agreement with the certified value of the standard reference material.
Conclusion
The proposed solid phase extraction procedure based on TiO2 functionalized with pyridine group shows a good selectivity for preconcentration and determination of palladium ions in trace levels. The low detection limit, palladium at trace level can be determined by this rapid and selective proposed method.
References
- Matthey J., Platinum 2008: 1 (2008).
- C. Hagelüken, Metall. 1-2: 31 (2006).
- Mehran Raizian, Naser Montazeri and Esmaeil Biazar, Orient J. Chem., 27(3): 203- 219 (2012).
- Spivakov B. Y., Malofeeva G. I. and Petrukhin O. M., Anal. Sci., 22: 503 (2006).
- Bulut V. N., Gundogdu A., Duran C., Senturk H. B., and Soylak M., J. Hazard. Mater., 146: 155 (2007).
- Thurman E.M., and Mills M.S. Solid-Phase Extraction: Principles and Practice, Wiley, New York (1998).
- Kiyoyama S., Yonemura S., Yoshida M., Shiomori K., Yoshizawa H., Kawano Y. and Hatate Y., React. Funct. Polym., 67: 522 (2007).
- Ghaedi M., Rezakhani M., Khodadoust S., Niknam K. and Soylak M., Scientific World Journal. doi:10.1100/2012/764195 (2012).
- Lotfi Zadeh Zhad H. R., Aboufazeli F., Sadeghi O., Amani V., Najafi E. and Tavassoli N., http://dx.doi.org/10.1155/2013/482793, (2013).
- Zhaoa X. N., Shia Q. Z., Xieb G. H., Zhoua Q. X., Chin. Chem. Lett., 19: 865 (2008).
- Ali Moghini and Mohamad Javad Poursharifi, Orient J. Chem., 28(1): 203-219 (2012).
- Hoogboom J., Garcia P. M. L., Otten M. B., Elemans J. A. A. W., Sly J., Lazarenko S. V., Rasing T., Rowan A. E., Nolte R. J. M., J. Am. Chem. Soc. 127: 11047 (2005).
- Fan Z., Jiang Z., Yang F., Hu B., Anal. Chim. Acta 510: 45 (2004).
- Tasviri M., Rafiee-Pour H. A., Ghourchian H., Gholami M. R., functionalized A., Appl. Nanoscience 1: 189 (2011)






