Determination of Cadmium (II) Ions in Environmental Samples : A Potentiometric Sensor
Mohammad Karimi1 * , Forouzan Aboufazeli1 , Hamid Reza Lotfi Zadeh Zhad1 , Omid Sadeghi1 and Ezzatollah Najafi1
1
Department of Chemistry,
Shahr-e-Rey Branch,
Islamic Azad University,
Tehran,
Iran
DOI: http://dx.doi.org/10.12944/CWE.7.2.02
Copy the following to cite this article:
Karimi M, Aboufazeli F, Zhad H.R.L.Z, Sadeghi O, Najafi E. Determination of Cadmium (II) Ions in Environmental Samples : A Potentiometric Sensor. Curr World Environ 2012;7(2):201-206 DOI:http://dx.doi.org/10.12944/CWE.7.2.02
Copy the following to cite this URL:
Karimi M, Aboufazeli F, Zhad H.R.L.Z, Sadeghi O, Najafi E. Determination of Cadmium (II) Ions in Environmental Samples : A Potentiometric Sensor. Curr World Environ 2012;7(2):201-206. Available from: http://www.cwejournal.org/?p=2739
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2012-07-12 |
|---|---|
| Accepted: | 2012-09-17 |
Introduction
Cadmium is a trace heavy metal of great importance in environmental protection since it is a highly toxic element.1 Determination of cadmium in environment samples is so important as this element exist in environment samples as a contaminant originating from industrial or urban waste pollution. As a result of high toxicity even at low concentrations, and various matrix interferences in real samples, developing an accurate, precise and selective method for cadmium determination is necessary. Different instrumental methods such as flame atomic absorption spectrometry (FAAS),2 graphite furnace atomic absorption spectrometry (GFAAS),3 inductively coupled plasma atomic emission spectrometric (ICP-AES),4 and electrochemical methods5 have been used for cadmium determination. Among these methods, potentiometric methods using ion sensors are common due to their accuracy, high rate, low cost and also being non-destructive.6 Potentiometric carbon paste electrodes, in comparison to polymeric membrane electrodes, posses very attractive properties such as ease of preparation, renewable surface, stability of their response, low ohmic resistance and no need of internal solution.7 In this technique, a chemical modifier is introduced to carbon paste electrode to increase the methods sensitivity.8 In carbon paste methods, carbon nano-tubes have attract lots of attention as a modifier due to having high electrical conductivity, high mechanical and thermal stability.9 These nano-tubes can be easily modified with a ligand in order to change their selectivity toward a specific ion. In this work, multi-walled carbon nanotubes were functionalized by dithizone. The material was characterized by FT-IR, SEM and elemental analysis. A carbon paste sensor was modified by this material and used for determination cadmium content in environmental samples. The effective parameters on response of the electrode were investigated and good selectivity toward cadmium ion was observed. This electrode can be used as fast simple method for determination of cadmium content in environmental samples with low concentrations.
Material and Methods
Regents and solutions
All reagents were of analytical grade and used without any further purification. Paraffin oil and Cadmium nitrate were purchased from Sigma-Aldrich Company. Carboxyl modified multiwalled carbon nanotube (COOH-MWCNT) was purchased from Neutrino Company (Tehran-Iran). Multiwalled carbon nanotubes were 30 µm length and 5-10 nm in diameter. The other chemicals such as Oxalyl chloride and dithizone were from Merck Company. All solutions were made using deionized water. The deionized water was provided from a Milli-Q (Millipore, Bedford, MA, USA) purification system.
Preparation of dithizone functionalized multiwalled carbon nanotube
For synthesis of dithizone functionalized multiwalled carbon nanotube, 1.0 g of COOH-MWCNT was suspended in 50 mL of dried CH2Cl2 under nitrogen atmosphere. Then 5 mL of oxalyl chloride was slowly added to mixture from a dropping funnel. After stirring for 24 h, CH2Cl2 was removed under reduced pressure, and the residue was suspended again in 50 mL of dried methanol. Then 5 mL triethylamine and excess amount of dithizone (2 g) were added to reaction mixture. After refluxing the mixture for 24 h, methanol was removed under reduced pressure and the sorbent was dried at 80 °C under vacuum. The formation of dithizone functionalized multiwalled carbon nanotube was confirmed by IR spectroscopy, elemental analysis and SEM micrograph. A schematic diagram of this synthesis is represented in Fig. 1.
Apparatus
The reference electrode was a glass cell, consisted of an R684 model Analion Ag/AgCl double junction. A Corning ion analyzer 250pH/mV meter was used for the potential measurements. The pH meter was a digital WTW Metrohm 827 Ion analyzer (Switzerland) equipped with a combined glass-calomel electrode. The pH adjustments were made at 25±1â—¦C. The elemental analyses (CHNS) were performed on a Thermo Finnigan Flash-2000 microanalyzer (Italy). IR spectra were recorded on a Bruker IFS-66 FT-IR Spectrophotometer. The SEM micrograph was recorded by a Vega-TeScan scanning electron microscope.
Preparation of modified carbon paste electrode
By thoroughly mixing an accurate of amount of graphite 67% graphite powder, paraffin 23%, 10% modified MWCNTs (W/W) the carbon paste electrode was prepared. The electrode body was fabricated from a glass tube of i.d. 5 mm and a height of 3 cm. To avoid possible air gaps, the paste was packed carefully into the tube tip, often enhancing the electrode resistance. A copper wire was inserted into the opposite end to establish electrical contact. The external electrode surface was smoothed on a soft paper. A new surface was produced by scraping out the old surface and replacing the carbon paste.
Electrode conditioning and Emf measurements
The electrode surfaces were conditioned in a solution of 1.0×10−4 mol L-1 Cd(NO3)2 and 1.0×10−3 mol L-1 NaNO3 for 24 hours. The electrodes were rinsed by deionized and polished before potentiometric measurements. In all solutions the potential was measured versus Ag, AgCl(s) reference electrode. The electrochemical cell can be represented as follows: Ag, AgCl (s), KCl (3 mol L-1) || analyte solution | carbon paste electrode
Sample preparation
The soil standard reference material was digested in an 8 mL mixture of 5% aqua regia with the assistance of a microwave digestion system. Digestion was carried out for 2 min at 250 W, 2 min at 0 W, 6 min at 250 W, 5 min at 400 W and 8 min at 550 W, and the mixture was then vented for 8 min and the residue from this digestion was then diluted with deionized water.10
Results and Discussion
Dithizone functionalized multiwalled carbon nanotube characterization
The reaction of chlorine group in acyl chloride with amine group in dithizone group leads to formation of this composite. A schematic diagram of this synthesis is represented in Fig 1. The formation of dithizone functionalized multiwalled carbon nanotube was confirmed by IR spectroscopy, elemental analysis and SEM micrograph. IR spectrum of this composite is as follow; IR (KBr, cm-1): 3400 (NH), 3017 (CH, aromatic), 2964 (CH, aliphatic), 1563 (C=C, aromatic), 1318 (C=S), 1237 (N=N) and 890 (MWCNT). In order to investigate the amount of grafted dithizone, elemental analysis was performed on this composite. According to elemental analysis results (%C=18.12, %H=1.81, %N=5.27, %S= 2.99), the dithizone concentration on the surface of this composite is approximately 0.94 mmol g-1. Finally, in order to investigate the morphology and size of this composite, SEM micrograph was performed on this modified MWCNT. According to the SEM micrograph, the nano-structure of MWCNT remained unchanged after functionalization and the multiwalled carbon nanotubes have approximately 20 nm diameter (Fig. 2).
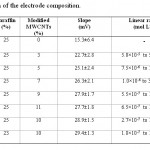 |
Table 1: Optimization of the electrode composition Click here to View table |
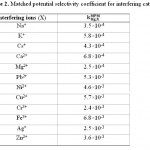 |
Table 2: Matched potential selectivity coefficient for interfering cations Click here to View table |
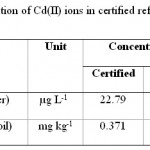 |
Table 3: Recovery of determination of Cd(II) ions in certified reference materials Click here to View table |
Electrode composition
The electrode composition is the most important factor in the responses and selectivity of the electrode. Different amounts of graphite powder, paraffin oil and modified MWCNTs were thoroughly mixed and the responses are listed in Table 1. In the first study no modifier was added to the electrode and only graphite powder, paraffin oil were used (electrode no. 1). In the next electrodes different amounts of modified MWCNTs were added to the electrode (electrode no. 2-7). It was observed that the electrode performance can improve by adding modified MWCNTs. This should be result of two factors: 1) improving the conductivity of the electrode which is the result of high conductivity of MWCNTs; 2) complexation of cadmium ion with dithizone which increases the analyte concentration on the surface of the electrode. The response of the electrode was increased with adding modified MWCNTs up to 10% (electrode no. 7) and in higher values the Nernstian slope was decreased (electrode no. 6). By changing the composition ratio to 67% graphite powder, paraffin 23%, 10% modified MWCNTs in electrode no. 8 the best results were ontained. A Nerstian solpe of 29.4 mV in a linear range of 1.8×10-7 to 1.0×10-4 mol L−1 was obtained. The standard deviation for ten replicates was 1.3 mV
Calibration curve
Quantitative determination of cadmium (II) ions was done by a calibration curve in the linear range of 1.8×10-7 to 1.0×10-4 mol L−1 versus Emf measurements. The calibration curve is shown in Fig. 3. The detection limit of the electrode was calculated by extrapolating the linear parts of the ion selective calibration curve.11,12 The limit of detection of the electrode was 1.0×10−7 mol L−1.
Influences of pH
The effect of pH of the test solution on the sensor potential was investigated by following the potential variation of the sensor over the pH range of 2.0 to 9.0. The pH of a sample solution of 1×10-5 mol L-1 of cadmium (II) ion was adjusted by introducing small drops of hydrochloric acid solution (0.10 mol L-1) and/or sodium hydroxide solution (0.10 mol L-1). The result of this study is shown in Fig. 4. The results show that the potentials of the sensor remain constant from pH of 3.0 to 7.0. Under more acidic conditions, the ligand may be protonated and thereby lose its capacity to form a complex with the metal ions, whereas for the higher pH values , the hydroxyl ions in the solution react with Cd(II) to make Cd(OH)2.
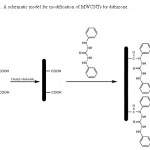 |
Figure 1: A schematic model for modification of MWCNTs by dithizone Click here to View figure |
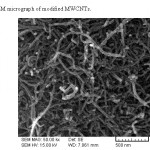 |
Figure 2: SEM micrograph of modified MWCNTs Click here to View figure |
Study of Response time
The average static response time was defined as the required time for the sensors to reach a potential of 90% of the final equilibrium values, after successive immersions in a series of solutions, each having a 10-fold concentration difference.11,12 To investigate this parameter, The Cd(II) concentration was changed in the liner range and the results were studied. The results showed that the response time for the proposed electrode is 37 seconds.
Influence of interference ions
The selectivity behavior is obviously one of the important characteristics of membrane sensors in which the possibility of reliable measurement of the target sample is determined. Matched potential method (MPM) is the recommended method for studying Influence of interferences ions in ion selective electrodes by IUPAC.13 The method is base on measuring the specific activity of the primary ion which is added to a reference solution. In this study the interfering ions were successively added to an identical reference solution with concentration of 1.0×10-6 mol L-1, until the measured potential matched to obtained value before adding the primary ions. Then matched potential selectivity coefficient,
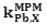 is calculated from the resulting primary ion to the interfering ion activity ratio,
is calculated from the resulting primary ion to the interfering ion activity ratio, 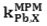 .14 The interference of Na+, K+, Cs+, Ca2+, Mg2+, Pb2+, Ni2+, Cu2+, Cr3+, Fe3+, Ag+ and Zn2+ was investigated and showed that they have no significant effect on the response to Cd2+. The
.14 The interference of Na+, K+, Cs+, Ca2+, Mg2+, Pb2+, Ni2+, Cu2+, Cr3+, Fe3+, Ag+ and Zn2+ was investigated and showed that they have no significant effect on the response to Cd2+. The 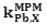 values for the interferences are shown in Table 2. The electrode showed a good selectivity toward Cadmium (II) ions.
values for the interferences are shown in Table 2. The electrode showed a good selectivity toward Cadmium (II) ions.
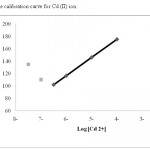 |
Figure 3: The calibration curve for Cd (II) ion Click here to View table |
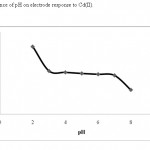 |
Figure 4: Influence of pH on electrode response to Cd(II) Click here to View table |
Lifetime
The lifetime of an electrode is the period of time that the electrode shows no changes in the efficiency of the measurements. The electrode was calibrated periodically with standard cadmium solutions. The next time the electrode was calibrated in the next week. It was observed that within 12 weeks no changes in the electrode response occur. After 12 weeks the sole was changed and linger range became more limited.
Method validation
Different type of standard reference materials (water and soil) was used for validation of this method. The samples were digested by mentioned method and the Cd(II) contents were analysed by this method. As it can be seen in Table 3, the results have good compatibility with certified ones and this method can be consider as an accurate and reliable method for cadmium determination in environmental samples.
Conclusion
A paste electrode was developed for determination of cadmium ions. . The electrode composition was 67% graphite powder, paraffin 23%, 10% modified MWCNTs (W/W). Effects of electrode composition, and pH on the electrode response were studied. The electrode has a long lifetime and a response time. A good selectivity to cadmium ion was observed which makes the electrode a good candidate for determination of cadmium content in environmental samples. Method validation was done by analysis of standard reference materials with a matrix of water and soil.
References
- Bowen H. J. M., Environmental chemistry of the elements, Academic Press, London, (1979).
- Xiang G., Wen S., Wu X., Jiang X., He L. and Liu Y., Food Chem., 132, 532 (2012). http://dx.doi.org/10.1016/j.foodchem.2011.10.053
- Maranhão T. A., Martendal E., Borges D. L. G., Carasek E., Welz B. and Curtius A. J., Spectrochim. Acta B, 62, 1019 (2007). http://dx.doi.org/10.1016/j.sab.2007.05.008
- Boevski I., Daskalova N. and Havezov I., Spectrochim. Acta B, 55, 1643 (2000).
- Wu K., Hu S., Fei J. and Bai W., Anal. Chim. Acta, 489, 215 (2003). http://dx.doi.org/10.1016/S0003-2670(03)00718-9
- Khan A. A. and Paquiza L., Desalination, 272, 278 (2011). http://dx.doi.org/10.1016/j.desal.2011.01.039
- Zhang T., Chai Y., Yuan R. and Guo J., Anal. Methods, 4, 454 (2012). http://dx.doi.org/10.1039/c2ay05668b
- Mashhadizadeh M. H., Khani H., Foroumadi A. and Sagharichi P., Anal. Chim. Acta, 665, 208 (2010). http://dx.doi.org/10.1016/j.aca.2010.03.023
- Faridbod F., Ganjali M.R., Larijani B. and Norouzi P., Electrochim. Acta, 55, 234 (2009). http://dx.doi.org/10.1016/j.electacta.2009.08.044
- Sayar O., Lotfi Zadeh Zhad H.R., Sadeghi O., Amani V., Najafi E., Tavassoli N., Biol. Trace Elem. Res. http://dx.doi.org/10.1007/s12011-012-9467-9
- Gupta V. K., Singh A. K. and Gupta B., Anal. Chim. Acta, 575, 198 (2006). http://dx.doi.org/10.1016/j.aca.2006.05.090
- Ganjali M. R., Norouzi P., Faridbod F., Yousefi M., Naji L., Salavati-Niasari M., Sensor Actuat. B-Chem., 120, 494 (2007). http://dx.doi.org/10.1016/j.snb.2006.03.002
- Buck P. R. and Lindner E., Pure Appl. Chem., 66, 2527 (1994). http://dx.doi.org/10.1351/pac199466122527
- Umezawa Y., Umezawa K., Hamada N., Aoki H., Nakanishi J. U. N., Sato M., Ping Xiao K. and Nishimura Y., Pure Appl. Chem., 48, 127 (1976). http://dx.doi.org/10.1351/pac197648010127






