Assessment of arsenic, nitrate and phosphorus pollutions in shallow groundwater of the rural area in Kurdistan province (Iran)
Zahed Sharifi1 * and Ali Akbar Safari Sinegani1
1
Department of Soil Science,
College of Agriculture,
Bu-Ali Sina University,
Postal Code,
6517833131
Hamedan
Iran
DOI: http://dx.doi.org/10.12944/CWE.7.2.07
Copy the following to cite this article:
Sharifi Z, Sinegani A.A.S. Assessment of Arsenic, Nitrate and Phosphorus Pollutions in Shallow Groundwater of the Rural Area in Kurdistan Province (Iran). Curr World Environ 2012;7(2):233-241 DOI:http://dx.doi.org/10.12944/CWE.7.2.07
Copy the following to cite this URL:
Sharifi Z, Sinegani A.A.S. Assessment of Arsenic, Nitrate and Phosphorus Pollutions in Shallow Groundwater of the Rural Area in Kurdistan Province (Iran). Curr World Environ 2012;7(2):233-241. Available from: http://www.cwejournal.org/?p=2806
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2012-07-12 |
|---|---|
| Accepted: | 2012-09-17 |
The rule of water quality on human health is well known and recently attracted a great deal of interest. Many water quality problems have been identified and addressed in the past from several parts of the world.1 According to Nature (2010) about 80% of the world's population (4.8 billion in 2000) lives in areas with threats to water security.2 Most cases of waterborne diseases and related deaths occur in developing nations are directly due to unsafe water, unsanitary conditions and insufficient hygiene.3,4
Shallow groundwater provides drinking water for human in most parts of the world including Iran. But, the water table of shallow groundwater is often quite near the surface. Therefore, there are a lot of risks for this groundwater both on its quantity and quality. In some areas groundwater resources are at risk from the results of point and non-point source pollutants such as agricultural fertilizer application, irrigation return flows, industrial and wastewater discharges, animal waste and household chemicals run-off, failing septic systems, etc.5-7
Kurdistan, a western province of Iran, is facing the problem of As contamination with geologic origin. The discovery of As in the groundwater of Kurdistan is a major concern to people's livelihood in the province. Exposure to high doses of As can cause organ cancers, organ damage, weakness, neural disorders and decreased appetite.1,8 Qorveh Plain is one of the most important agriculture areas in the Kurdistan province. However, the water and fertilizers in this area are not used effectively and economically. Thus, arsenic pollution along with agricultural drain waters from the heavy fertilizer lands is a great challenge to water recourses in this area. No scientific and systematic studies have been conducted in the region. However, several studies have documented that contamination (e.g. nitrate) of household and farm wells can occur from agricultural activities around the wells.7, 9-11 In order to improvement in water supplies and sanitation, the monitor and assessment of water quality on regular basis is very important. Hence, the present work is undertaken with the objective to assess shallow groundwater quality for drinking purpose in the rural area of Kurdistan province (Iran).
Material and Methods
Study Area
Seven villages of Qorveh plain located around the Sari Gunay Gold Mine were selected for this study. These villages confined between longitudes 47˚ 57׳ 40״ and 48˚ 8׳ 34״ E and latitudes 35˚ 7׳ 2״ and 35˚ 12׳ 47״ N (Figures, 1a, 1b and 1c) in the northern-east region of Qorveh city in Kurdistan province, western Iran. The climate in this area is semi-arid and the average annual rainfall and temperature is 339 mm and 11.4 oC, respectively. Twenty-five shallow groundwater samples were collected from the area during September 2009. Of all these samples, 14 were collected from household wells (depth on average, 11 m) including Babashydolah (B1), Dashkasan (D1 to D6), Dosar (S), Jodaqye (G1 to G3), Narenjak (N) and Nayband (A1 to A3), the other 11 (depth on average, 26 m) were collected nearly from all shallow farm wells in this area including Babashydolah (B2 and B3), Dashkasan (D7 to D9) and Zang Abad (Z1 to Z5) (Table 1).
Sampling Method
To collect the water samples, 300 ml (for assessment of cations) and 1000 ml (for assessment of anions plus pH and electrical conductivity (EC) clean polyethylene containers were washed by detergent, rinsed first with hot water, then once with 0.1 N HCl and twice with distilled water. Then containers were left to dry, and then they were capped. The containers were then ready to be used to collect the water samples from the wells. Water samples were collected from wells, taps or other points used by local residents. The samples were collected after at least 10 min of pumps and taps operation. To keep the cations as solution and prevent adsorption or deposition on the walls of the sample containers, pH of the smaller containers was reduced to below 2 using ultra pure HNO3 immediately after filtering. After the sampling, the samples were immediately transferred to laboratory and refrigerated (at 4 ËšC) until their analysis.
Sample Analysis
Samples were analyzed in the laboratory for the major physio-chemical properties according to the Standard Methods for the Examination of Water and Wastewater (volume 1) described in Andrew et al. (2005) [12]. The pH and water electrical conductivity (ECw) were measured on pH and electrical conductivity meters, respectively. Calcium (Ca2+) and magnesium (Mg2+) were determined by complexometric method. Chloride (Cl–) was measured by AgNO3 titration method. Bicarbonate (HCO3–) was determined by titration with H2SO4. Sodium (Na+) and potassium (K+) were measured by flame emission photometric method. Sulphate (SO42–) was determined by turbidimetic method. Silicon (Si) was measured by the spectrophotometric molybdosilicate method. Nitrate (NO3–) and phosphorus (P) were measured by spectrophotometric method. Total arsenic (Astotal) was determined by the graphite furnace atomic absorption spectrophotometry (GF-AAS) (Varian 220, Mulgrave, Victoria, Australia) and the total iron (Fetotal) and total manganese (Mntotal) were also determined using atomic absorption spectrophotometry (AAS). Total hardness (TH) was calculated as CaCO3. Total dissolved solids (TDS) were calculated by using the following equation:
TDS (ppm) = 640 × ECw (dSm–1).
Results and Discussion
The physicochemical analyses of the household and farm wells were statistically analyzed and the results are presented in Tables 2 and 3 respectively. In this study, assessment of the suitability of collected samples for human consumption was evaluated by comparing the physicochemical parameters with standard set of the World Health Organization (WHO 2011a).4 The results have been discussed by the following basic criteria (Tables 2 and 3):
pH
The pH values of the water samples ranged between 6.0 to 7.7 at household and 5.9 to 7.4 at farm wells. On average, water sampled from household wells (7.2, weakly alkaline) had comparatively higher pH contents than those sampled from farm wells (6.5, acidic). Lower pH values in the farm wells may be attributed by larger quantities of dissolved minerals13 and acidic ions such as SO42– due to the cropping activity (use of fertilizers, pesticides, etc).14 It confirm by the higher amounts of SO42– and TDS at the farm wells than the household wells (Tables 2 and 3). In the current study because of acidic pH values of farm wells 54% of the samples go beyond the normal permissible range of pH (6.5-8.5) for drinking usage. However, 14% of household wells did not fall in this desirable range. Waters with a low pH are corrosive, which can damage to metal pipes and other fixture of the plumbing system. The problem is more acute when the waters contact toxic metal piping systems where these metals such as copper, lead, zinc, etc, can dissolve into the human’s drinking water.
Arsenic
The deleterious effect of heavy metals in the environment is well known.15 Total As concentrations ranged from 15.6 to 60.5 ppbin household wells, 47.4 to 102.4 ppbin farm wells. It is a major concerning that all water samples from household and farm wells showed As concentrations of above the WHO guideline value in potable water (10 ppb),4 while 91% of farm wells and 21% of household wells exceeded the maximum acceptable level in potable water in Iran (50 ppb).16 High concentration of As in the drinking water can have detrimental effects on health. It is worth to note that some multi-chronic arsenical poisoning symptoms, such as skin lesions (including, keratosis and pigmentation), and even amputation due to gangrene, have been reported among residents in west of Iran.1,8 In this area, as in many part of the world, naturally-occurring As is responsible for groundwater contamination. It is well known that natural enrichment of groundwater by As is governed by the geophysical, chemical and biological processes, such as oxidation–reduction, dissolution–precipitation and sorption–desorption [17]. An important observation in this study is that As contamination was increased with depth of wells (r = 0.71; P<0.01) and its concentration in farm wells (on average, 70.8 ppb) was nearly 2 times higher than household wells (on average, 36.8 ppb). In fact, in household wells, As concentrations in 79% of water samples stay below 50 ppb because As in the oxic shallow groundwater, and in recharging water, is sorbed to aquifer sediments.18
 |
Table 1: Identification of sampling wells Click here to View table |
Iron and Manganese
Total iron and Mntotal concentration varied from 0 to 0.23 and 0 to 0.33 ppm in household wells, and 0.03 to 1.86 and 0 to 0.12 ppm in farm wells, respectively. Out of all wells sampled, D7 and D9 (from farm wells) contain Fetotal higher than allowable limit (0.3 ppm) for drinking purpose.4 Eight percent of water samples i.e. D5 (from household wells) and D9 (from farm wells) showed Mntotal concentrations above the allowable limit (0.1 ppm) for drinking usage.4
Nitrate and Phosphorus
The concentrations of NO3– varied from 23.2 to 916.9 ppm at household wells, 1.8 to 79.0 at farm wells with a mean of 178.3 and 38.2 ppm, respectively. In compared to the WHO’s drinking water guideline of 50 ppm for NO3−, 71% of household wells and 45% of farm wells showed higher concentrations.4 The high concentration of nitrate in the surveyed groundwaters is toxic and can cause methemoglobimia or blue-baby syndrome in infant and also can increase the risk of gastric cancer.19 In compared to farms wells, concentrations of nitrate at household wells were unusually high (on average 178.3 ppm). It can be as a result of closeness of septic tank to the wells.20, 21 lower depth and higher pH of the wells15 and abandoned livestock yards in the rural area. In addition to all the aforementioned, most of the household wells often left open that exposes the wells to contamination by runoff during heavy precipitation.
The concentration of phosphorus was between 0.02 to 0.19 ppm at household wells, 0.05 to 0.17 ppm at farm wells. There is no guide line for phosphorous in drinking water, but phosphorus concentrations in all of the water samples were considerably higher than the normal limit of phosphorus (0.02 ppm) in shallow groundwater.22
It is possible that the high concentration of nitrate and phosphorous in these groundwaters result from excessive application of manure and inorganic fertilizer at a rate greater than agronomic rate in this area. Farmer inquiries indicate that in addition to chemical fertilizer – used often up to 2–3 times the recommended rate – the use of organic manure, especially poultry manure, the type most frequently used (for potato fields about 10 ton ha-1year-1 is used). Nitrate and phosphorus from such sources coupled with widespread irrigation can be increased groundwater contamination via runoff and infiltration in this area as previously shown by Jeyaruba and Thushyanthy (2009)23 and Jalali (2005 and 2009).10,11
 |
Table 2: Summary statistics of physicochemical analysis and wise suitability categorization of them for drinking in household wells collected in the rural area of Qorveh plain (unit as ppm except As (ppb) and Ecw (dSm-1) Click here to View table |
Chloride
The concentrations of Cl– ion lie in between the ranges of 34.5 to 469.0 and 51.5 to 202.7 with a mean of 139.9 and 112.0 ppm, at household and farm wells, respectively. The concentration of Cl– at all of farm wells were well within the acceptable drinking limit values for Cl– (250 ppm),4 however, 14% of household wells i.e. A1 and D3 exceeded the recommended level.
 |
Table 3: Summary statistics of physicochemical analysis and wise suitability categorization of them for drinking in farm wells collected in the rural area of Qorveh plain (unit as ppm except As (ppb) and Ecw (dSm-1) Click here to View table |
Bicarbonate and Sulphate
The concentrations of HCO3– and SO42– varied from 201.0 to 461.5 and 37.8 to 309.7 ppm at household, 397.4 to 958.3 and 85.3 to 331.1 ppm at farm wells, respectively. Fourteen percent of household wells and 9% of farm wells were beyond the permissible limit (250 ppm) for SO42– .4 Although the amount of this ionat Z2 (176.7 ppm), Z3 (192.3 ppm) and Z4 (213.8 ppm) was considerable.
Sodium, Calcium and Magnesium
The concentrations of the Na+, Ca2+ and Mg2+ ranged from 41.0 to 169.6, 52.7 to 468.9 and 7.3 to 125.4 ppm, with the respective average values 91.4, 152.0 and 38.1 ppm at household wells, 91.2 to 151.8, 117.2 to 253.2 and 7.3 to 64.2 ppm with the respective average values 122.2, 185.0 and 33.0 ppm, at farm wells. As shown in Tables 2 and 3 all of water samples under test are well within the acceptable drinking limit values for Na+ (200 ppm) and Mg2+ (150 ppm).4 However, 14% of household wells i.e. D3 and D4 and 45% of farm wells i.e. S, Z1 and Z3 to Z5 are exciding the maximum permissible level for Ca2+ (200 ppm) in drinking water.4
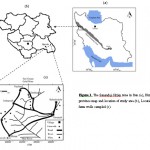 |
Figure 1: The Sanandaj-Sirjan zone in Iran (a), Kurdistan province map and location of study area (b), Location of farm wells sampled (c) Click here to View figure |
Silica and Potassium
The Si and K+ concentrations varied from 16.2 to 37.5 and 1.0 to 30.4 ppm, with the respective mean values of 23.8 and 5.9 ppm at household wells, 19.2 to 35.4 and 7.7 to 28.2 ppm, with the respective mean values 26.0 and 11.7 ppm at farm wells. Permissible limit for silica in drinking water have not been prescribed not only by the WHO but also by similar agencies. However, in view of the high concentration of Si in the Earth’s crust (28% by weight); life would have been real precarious if excessive ingestion of Si is really harmful. Further research is required in this direction. In the case of potassium, although potassium may cause some health effects in susceptible individuals, potassium intake from drinking-water is well below the level at which adverse health effects may occur.24 Thus, there is no guideline for potassium.
Salinity
Salinity is the total amount of inorganic solid material dissolved in any natural water, and water salinization refers to an increase in total dissolved solids (TDS) and the overall chemical content of the water.25 Salinity of groundwater is a useful indicator of the land area and drinking water at risk from salinity. Electrical conductivity and TDS are used as tools for salinity assessment; their amounts ranged from 0.4 to 3.5 dSm–1 and 279.2 to 2217.3 ppm at household wells, 1.0 to 1.9 dSm–1 and 652.2 to 1223.4 ppm at farm wells, respectively. As shown in Tables 2 and 3, on average, the TDS at farms wells (944.4 ppm) was higher than household wells (826.5 ppm), it can be attributed to the grater effects of human activities such as application of fertilizers and irrigation practice on salinity of farm wells than the household wells in this area. Previous studies have shown that salinity is usually affected mainly by topography, lithology of aquifer, recharge, runoff and discharge conditions of groundwater.26 The palatability of water with a TDS level of less than about 600 ppm is generally considered to be good; all of farm wells and 71% of household wells were exceeded this desirable limit. However, Drinking-water becomes significantly and increasingly unpalatable at TDS levels greater than about 1000 ppm; 28% of household wells and 45% of farm wells exhibit TDS values outside the maximum permissible limit. The presence of high levels of TDS in these groundwaters can have an objectionable to consumers, owing to excessive scaling in water pipes, heaters, boilers and household appliances.4
Total Hardness (TH)
Water hardness is primarily due to the amount of calcium and magnesium and, to a lesser extent, iron. The TH value ranged from 162.0 to 1687.1 and 448.8 to 756.2 with an average of 536.5 and 597.8 ppm as-CaCO3, in household and farm wells, respectively. According to the grading standards of TH, all of farm wells and 71% of household wells fall in the very hard waters category (TH>300 ppm as-CaCO3). The recommended value of TH for potable water is 1000 mg as CaCO3. The TH of all water samples except one sample (D9) was well within the permissible limit. But previous studies have shown that consumption of waters with high TH cause numerous human diseases such as heart disease and kidney stone.27
Conclusion
The shallow groundwater sources in the rural area of Kurdistan province have been evaluated for their physicochemical composition and suitability for drinking purpose. Results showed that As, NO3– and P pollution are in an alarming state in this area. The observed As in these groundwaters has a geologic origin and the high NO3– and P could occur from human activities such as agriculture, household chemicals run-off and failing septic systems. All wells under test failed at least one safe drinking water standard. So that, based on Astotal, NO3–, TDS, pH, Ca2+, SO42– and Mntotal, 100%, 71%, 28%, 14%, 14%, 14%, and 7% of analyzed samples at household wells and 100%, 45%, 45%, 54%, 45%, 9%, and 9% of analyzed samples at farm wells were unsuitable for human consumption, respectively. Other parameters that exceeded WHO guideline values in this assessment were Fetotal (18% of farm wells) and Cl– (14% of household wells). In conclusion, in order to improve public health, the users of the groundwaters must be awareness on the dangers of consumption of the waters.
Acknowledgements
We acknowledge our gratefulness to the residents of all the villages we visited for their contribution to the research.
References
- Barati A. H. Maleki A. and Alasvand M., Multitrace elements level in drinking water and the prevalence of multi-chronic arsenical poisoning in residents in the west area of Iran, Sci. Total. Environ., 408: 1523-1529 (2010).
- Nature online Balancing water supply and wildlife, 29 September, http:// www.nature.com/news /2010/10 /100929 / full /news. 2010.505. html (2010).
- Olajire A. A. and Imeokparia F. E., Water quality assessment of Osun river: studies on inorganic nutrients. Environ. Monitoring Assess, 69(1): 17-28 (2001).
- WHO, Guidelines for drinking water quality, 2nd ed, World Health Organization Geneva, (2011a).
- Swistock B. R. Clemens S. and Sharpe. W. E., Drinking Water Quality in Rural Pennsylvania and the Effect of Management Practices, Report of the School of Forest Resources and Institutes of Energy and the Environment Pennsylvania State University, (2009).
- Mitra B. K. Sasaki C. Enari K. Matsuyama N. and Fujita M., Suitability assessment of shallow groundwater for agriculture in sand dune area of northwest Honshu Island, Appl. Ecol. Environ. Res., 5(1): 177-188 (2007).
- Aelion C. and Conte B., Susceptibility of residential wells to VOC and nitrate contamination, Environ. Sci. Technol., 38: 1648-53 (2004).
- Mosaferi M. Yunesian M. Dastgiri S. Mesdaghinia A. R. and Esmailnasab N., Prevalence of skin lesions and exposure in drinking water in Iran, Sci. Total. Environ., 392(1): 69-76 (2008).
- Feldman P. R. Rosenboom J. W. Saray M. Navuth P. Samnang C. and Iddings S., Assessment of the chemical quality of drinking water in Cambodia, J. Water Health., 5(1): 101-116 (2007).
- Jalali M., Nitrates leaching from agricultural land in Hamadan western Iran, Agr. Ecosyst. Environ., 110: 210-218 (2005).
- Jalali M., Phosphorous concentration solubility and species in the groundwater in a semi-arid basin southern Malayer western Iran, Environ. Geol., 57: 1011-1020 (2009).
- G.S. Kalwania and R. Shyam, Orient J. Chem, 28(1): 547-552 (2012).
- B.Y. Kamaruzzaman, M.S. Zahir, B.A. John, K.C.A. Iqbal and J.S. Goddard, Orient J. Chem, 27(2): 505-510 (2011).
- Andrew D. Lenore S. Eugene W. and Arnolr E., Standard Methods for the Examination of Water and Wastewater, 21th edition, American Public Health Association/ American Water Works Association/Water Environment Federation Washington DC (2005).
- Napacho Z.A. and S.V. Manyele., Quality assessment of drinking water in Temeke District (part II): Characterization of chemical parameters, Afr. J. Environ. Sci. Technol., 4(11): 775-789 (2010).
- Liu A. Ming J. and Ankumah R. O., Nitrate contamination in private wells in rural Alabama United States, Sci. Total. Environ., 346(1-3): 112-120 (2005).
- Sharma R. K. and Agarwal M., Biological effects of heavy metals. J. Environ. Biol., 26: 301-313 (2005).
- Iranian Industrial Research and Standard Association Natinal standard of drinking water, Report of the Iranian Industrial Research, No, 1053 (1999).
- Wang S. and Mulligan C. N., Natural attenuation processes for remediation of arsenic contaminated soils and groundwater, J. Hazard. Mater., 138: 459-470 (2006).
- Nickson R. T. McArthur J. M. Shrestha B. KyawMyint T. O. and Lowry D., Arsenic and other drinking water quality issues Muzaffargarh District Pakistan, Appl. Geochem., 20(1): 55-68 (2005).
- Fewtrell L., Drinking-Water Nitrate Methemoglobinemia and global burden of disease: a discussion, Environmental Health Perspective, 112: 1-5 (2004).
- Abdel-Lah A. K. and Shamrukh M., Impact of septic system on ground water quality in a nile valley village Egypt, Sixth International Water Technology Conference IWTC, Alexandria Egypt, (2001).
- Adejuwon Joseph O. and Mbuk Chritopher J., Biological and physiochemical properties of shallow wells in Ikorodu town Lagos Nigeria, J. Geol. Min. Res., 3(6): 161-168 (2011).
- Fuhrer G. J. Gilliom R. J. Hamilton P. A. Morace J. L. Nowell L. H. Rinella J. F. Stoner J. D. and Wentz D. A., The quality of our Nation’s waters: Nutrients and pesticides: Report of the U.S. Geological Survey Circular., 1225: 82 p (1999).
- Jeyaruba T. and Thushyanthy M., The Effect of Agriculture on Quality of Groundwater: A Case Study, Middle-East J. Sci. Res., 4(2): 110-114 (2009).
- WHO., Potassium in drinking-water, Background document for development of WHO Guidelines for Drinking-water Quality Series No. WHO/HSE/WSH/09.01/7, World Health Organization Geneva, (2009).
- Richter B. C. and Kreitler W. C., Geochemical techniques for identifying sources of groundwater salinization, CRC Press, New York, (1993).
- Peiyue L. Qian W. and Jianhua W., Groundwater Suitability for Drinking and Agricultural Usage in Yinchuan Area China, Int j Environ Stud., 1(6): 1241-1249 (2011).
- WHO., Hardness in Drinking water, Background document for development of WHO Guidelines for Drinking-water Quality, Series No.WHO/HSE/WSH/10.01/10/Rev/1. World Health Organization Geneva, (2011b).






