Synthesis, Characterization and Thermal Studies of a N, N’- bis(2- hydroxy –alpha- methyl benzylidene) Isobutyl Diamine Uranyl (VI) Nitrate [UO2 (HMBUD)]2+
Shahriar Ghammamy1 * and Sajjad Sedaghat1
1
Department of Chemistry,
Faculty of Science,
Imam Khomeini International University,
Qazvin
Iran
DOI: http://dx.doi.org/10.12944/CWE.7.1.15
N, N’- bis(2- hydroxy –alpha- methyl benzylidene) isobutyl diamineabbreviated as HMBUD was synthesized and characterized. N, N’- bis(2- hydroxy –alpha- methyl benzylidene) isobutyl diamineUranyl (VI) nitrateprepared by reaction of nitrate salt of UO2(NO3)2.6H2O with HMBUD. In this research, some of the inorganic complexes of uranyl with N- donor ligands were synthesized. Complexes were characterized by FT-IR and UV, ¹HNMR, ¹³CNMR spectra, TG/DTG measurements and some physical properties. The results of simultaneous TG-DTG-DTA analyses of the complexes show the final degradation product for these complexes are UO3. Also the results show chelation causes drastic change in the biological properties of the ligands and also the metal moiety. So the toxic effects of uranyl can be prevented by using chelating agent and complexation of the potentially multidentate ligands.
Copy the following to cite this article:
Ghammamy S, Sedaghat S. Synthesis. Characterization and Thermal Studies of a N, N’- bis(2- hydroxy –alpha- methyl benzylidene)Isobutyl Diamine Uranyl (VI) Nitrate [UO2 (HMBUD)]2+. Curr World Environ 2012;7(1):93-100 DOI:http://dx.doi.org/10.12944/CWE.7.1.15
Copy the following to cite this URL:
Ghammamy S, Sedaghat S. Synthesis. Characterization and Thermal Studies of a N, N’- bis(2- hydroxy –alpha- methyl benzylidene)Isobutyl Diamine Uranyl (VI) Nitrate [UO2 (HMBUD)]2+. Curr World Environ 2012;7(1):93-100. Available from: http://cwejournal.org?p=360/
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2011-12-20 |
|---|---|
| Accepted: | 2012-03-25 |
Introduction
The coordination chemistry of transition metals with ligands from the uranyl family hasbeen of interest due to different bonding modes shown by these ligands with both electron rich and electron poor metal. In principle, the central transition metal atoms of different soft and hard Lewis acidity usually need to be satisfied in the most suitable fashion.Schiff base metal complexes have been widely studied because they have industrial, antifungal, antibacterial, anticancer and herbicidal applications.
Nitrogen-containing ligands such as Schiff bases and their metal complexes played an important role in the development of coordination chemistry resulting in an enormous number of publications, ranging from pure synthetic work to physicochemical1 and biochemically relevant studies of metal complexes2–6 and found wide range of applications. Other kinds of nitrogen-containing ligands are well-known pyrimidine systems such as purine analogues that exhibit a wide range of biological activities. Fused pyrimidine compounds are valued not only for their rich and varied chemistry, but also for many important biological properties. Among them, the furopyrimidine ring system, because of a formal isoelectronic relationship with purine, is of special biological interest. It has numerous pharmacological and agrochemical applications, namely, antimalarials, antifolates, and antivirus, as well as potential radiation protection agents. Recently, some furopyrimidines were shown to be potent ascular endothelial growth factor receptor 2 (VEGFR2) and epidermal growth factor receptor (EGFR) inhibitors. Because of the importance of furo (2,3-d) pyrimidine derivatives, several methodologies for synthesizing them have already been developed. However, many of the synthetic protocols reported so far prolonged reaction times, harsh reaction suffer from disadvantages, such as relying on multistep reactinos, needing anhydrous conditions, low yields, use of metal- containing reagents, and special instruments or starting materials. Therefore, the development of new and efficient methods for the preparation of furo (2,3-d) pyrimidine derivatives is still strongly desirable.7 Pyrimidines represent a very interesting class of compounds because of their wide applications in pharmaceutical, phytosanitary, analytical, and industrial aspects, for example, as antibacterial, fungicide,8 antihelmintics, antitubercular, anti-HIV, antidegenerative and hypothermic activities,8 and herbicides , and have biological activities.9–13 It has long been known that metal ions involve in biological processes of life and have been subject of interest. The modes of action of these metal ions are often complex but are believed to involve bonding to the heteroatom of the heterocyclic residues of biological molecules, that is, proteins, enzymes, nucleic acids and so forth.14 From these points of view, it is interesting to study different types of transition metal complexes of these biologically active ligands. In this paper, the synthesis characterization, and antitumor properties of a number of the ligands and uranyl complexes have been studied. In this work, we report the synthesis and structural studies of the ligand and N, N’- bis(2-hydroxy –alpha- methyl benzylidene) isobutyl diamineUranyl (VI) nitrate.
Material and Methods
Solvents were purified bystandard methods. All reagents were supplied by Merck and were used without further purification. Melting point was determined in an Electro thermal 9200. The FT-IR spectra were recorded in the range 400–4000 cm-1 by KBr disk using a Bruker Tensor 27 M 420 FT-IR spectrophotometer. The UV–Vis spectra in CH3CN were recorded with a WPA bio Wave S2 100 spectrophotometer. Thermo gravimetric analyses were done on a Perkin Elmer TGA/DTA lab system l (Technology by SII) in nitrogen atmosphere with a heating rate of 20°C/min from 35- 700°C.¹ H and ¹³ C-NMR spectra were measured on a BRUKER DRX-500 AVANCE spectrometer at 500 MHz.
Synthesis of the [UO2 (HMBUD)]2+
For synthesis of the [UO2 (HMBUD)]2+to a magnetically stirred of ligand (0.45g, 1.4mmol) in acetonitrile(10ml) was added uranyl (VI) nitrate (0.69g, 1.4mmol) at room temperature. The reaction mixture was further stirred for 3 hours to ensure the completion and precipitation of the formed complex. The precipitated solid complex was filtered and washed several times with diethyl ether to remove any traces of the unreacted starting materials.Yield, 85%.Anal.Mp: 225 °C. ¹HNMR (DMSO): 6.8-8 (CH phenol), 2.7 (CH3), 3.1 (CH2), FT-IR (KBr, cm-1): 1287s (ν C-N), 1622 s (ν C=N), 2929 br(ν OH), 572 m (ν U-O), 464m (ν U-N), 921 s (ν O=U=O), UV-vis (DMSO): λmax 260nm(ε 22000), 320nm(å 10000), 410nm(å 3600)(Figure 1-9). [UO2 (HMBUD)]2+is soluble in acetone, DMF and DMSO and insoluble in water, methanol, Acetonitrile, dichloro methane, diethyl ether and hexane and little soluble in chloroform and ethanol.Figure 10, 11 shows Chemical structures of HMBUD and [UO2 (HMBUD)]2+.
 |
Figure 1: FTIR spectrum of HMBUD (KBr Disk) Click here to View figure |
![fig2 Fig. 2: FTIR spectrum of [UO2 (HMBUD)]2+(KBr Disk)](http://www.cwejournal.org/wp-content/uploads/2012/09/fig21-150x150.jpg) |
Figure 2: FTIR spectrum of [UO2 (HMBUD)]2+(KBr Disk) Click here to View figure |
 |
Figure 3: 1H- NMR spectrum of HMBUD Click here to View figure
|
Analysis of HMBUD Ligand
Anal:%69. Calcd of C20H24N2O2; C;74.09, H; 7.4, N; 8.63; found: C; 80.11, H. 8.20, N; 9.12. Mp192-194 °C, ¹HNMR (DMSO): 6.7-7.6 (CH phenol), 1.5 (CH3), 3.6 (CH2), 12.8 (OH), FT-IR (KBr, cm-1): 1309s (ν C-N), 1612 s (ν C=N), 2931br(ν OH), UV-vis (DMSO): λmax268nm(ε 28000), 355nm(ε 10000). HMBUDis soluble in acetonitrile, DMSO, chloroform, dichloro methane and diethyl ether and insoluble in acetone, water, methanol, ethanol and hexane and little soluble DMF.
![fig4 Fig. 4: 1H- NMR spectrum of [UO2 (HMBUD)]2+](http://www.cwejournal.org/wp-content/uploads/2012/09/fig41-150x150.jpg) |
Figure 4: 1H- NMR spectrum of [UO2 (HMBUD)]2+ Click here to View figure |
 |
Figure 5: 13C- NMR spectrum of HMBUD Click here to View figure |
Results
Preparation of Ligand and complex
In this paper, we report a new method of the synthesis of N, N’- bis(2- hydroxy –alpha- methyl benzylidene) isobutyl diamineUranyl (VI) nitrate. The compound was obtained by reaction of UO2(NO3)2.6H2O and HMBUD and was synthesized through a one-step reaction. Our procedure for producing compound has some advantages. For example, there is no side product in preparing [UO2 (HMBUD)]2+ in our method, the reaction is quite fast and does not require any severe conditions such as high pressure or high temperature, and it is not sensitive to air.Compounds were characterized by several techniques using FT-IR, UV-Visible and NMR spectra Thermal analysis were studied for these compounds. The [UO2 (HMBUD)]2+has225 °C melting points respectively. It is soluble in acetone, DMF and DMSO and insoluble in water, methanol, Acetonitrile, dichloro methane, diethyl ether and hexane and little soluble in chloroform and ethanol. The spectral data of the complexes have good relationship with the literature data.The IR spectra of the Schiff base show characteristic bands due to ν(OH), ν(C=N) and ν(C-N) in the region 2931cm-1, (1612, 1309) cm-1respectively. The strong band in the region 1612, 1309cm-1 in the IR spectra of the Schiff base are assigned to ν( C=N), ν( C-N) respectively. In the case of U(VI) complex we observed the following changes. The bands appeared around 572, 464, 921, 1287, 1622, 2929cm-1 due to ν(U-O), ν(U-N), ν (O=U=O), ν (C-N), ν(C=N), ν (OH) IR spectra of ligand (HMBUD) show a broad medium intensity band in the region 2931cm-1due to OH.
 |
Figure 6: UV/ Vis spectrum of HMBUD(DMSO, 5×10-4 M) Click here to View figure |
![fig7 Fig. 7: UV/ Vis spectrum of [UO2 (HMBUD)]2+(DMSO, 5×10-4 M)](http://www.cwejournal.org/wp-content/uploads/2012/09/fig7-150x150.jpg) |
Figure 7: UV/ Vis spectrum of [UO2 (HMBUD)]2+(DMSO, 5×10-4 M) Click here to View figure |
![fig8 Fig. 8: Thermal analysis data of [UO2 (HMBUD)]2+](http://www.cwejournal.org/wp-content/uploads/2012/09/fig8-150x150.jpg) |
Figure 8: Thermal analysis data of [UO2 (HMBUD)]2+ Click here to View figure |
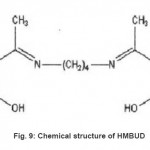 |
Figure 9: Chemical structure of HMBUD Click here to View figure |
![fig10 Fig. 10: Chemical structure of [UO2 (HMBUD)]2+](http://www.cwejournal.org/wp-content/uploads/2012/09/fig10-150x150.jpg) |
Figure 10: Chemical structure of [UO2 (HMBUD)]2+ Click here to View figure |
Thermo gravimetric analyses
The thermal properties of these compounds were investigated by thermo grams (TG, DTG andDTA). Figure 9shows TGA and DTA curves for[UO2 (HMBUD)]2+. In the temperature range from 300-410°C, 60% weight losing was observed which was related to the loss of most parts of compound.
Discussion
In this research, some of the inorganic complexes of uranyl with N- donor ligands were synthesized. Complexes were characterized by FT-IR and UV, ¹HNMR, ¹³CNMR spectra, TG/DTG measurements and some physical properties. The results of simultaneous TG-DTG-DTA analyses of the complexes show the final degradation product for these complexes are UO3.
Acknowledgments
We gratefully acknowledge the financial support from the Research Council of Imam KhomeiniIslamic Azad University and many technical supports that provided by TarbiatModarres University.
References
- VidmarR.J.IEEE Trans.Plasma Sci., 21: 876 (1992).
- Murthy A.S.N. andReddy A.R.Journal ofChemical Sciences, 90: 519 (1981).
- RazakantoaninaV.N.K. and Phung P. Parasitology Research,86: 665 (2000).
- Royer R.E. and Deck L.M. Journal of Medicinal Chemistry,38: 2427 (1995).
- Flack M.R. and Pyle R.G. The Journal of Clinical Endocrinology & Metabolism, 76:1019 (1995).
- BaumgrassR. andWeiwadM. Journal of Biological Chemistry, 276: 47914 (2001).
- TeimouriM.B. and Bazhrang R. Bioorganic & Medicinal Chemistry Letters, 16:3697(2006).
- TeimouriM.B. Tetrahedron, 62: 10849 (2006).
- GrevyJ.M.,TellezF. Inorganica ChimicaActa, 339: 532 (2002).
- BernalteA. andBarrosF. J. Polyhedron, 18:2907 (1999).
- Lemma K. andBerglund J. Journal of Biological Inorganic Chemistry, 5: 300 (2000).
- Campbell M.J.M. Coordination Chemistry Reviews,15: 279 (1975).
- Padhy´eS. andKauffman G.B. Coordination Chemistry Reviews, 63: 127 (1985).
- Erwin B. and OmoshileC. Journal of the Chemical Society Perkin Transactions, 2:1333 (1995).
- Zhao G. andLin H. Journal of Inorganic Biochemistry, 70: 219 (1998).






