Water Quality Index (W.Q.I.) of Pariyej Lake Dist. Kheda-Gujarat
F. J. Thakor1 * , D. K. Bhoi2 , H. R. Dabhi3 , S. N. Pandya1 and Nikitaraj B. Chauhan4
1
Department of Biology,
J. and J. College of Science,
Nadiad,
Dist. Kheda,
387 001
Gujarat
India
2
Department of Chemistry,
J. and J. College of Science,
Nadiad,
Dist. Kheda,
387 001
Gujarat
India
3
Department of Chemistry,
Navjivan Science College,
Dahod. Dist. Dahod,
Gujarat,
389 151
India
4
Department of Biology,
Ashok and Rita Institute of Integrated Study and Research in Biotechnology,
V.V. Nagbar,
Dist. Anand,
Gujarat
India
DOI: http://dx.doi.org/10.12944/CWE.6.2.19
The present study calculates the Water Quality Index (W.Q.I.) of Pariyej Lake and assesses the impact of industries, agriculture and human activities. Physico – chemical parameters were monitored for the calculation of W.Q.I. for the rainy, winter and summer seasons. The parameters namely PH, Total hardness, TDS, Calcium, Chloride, Nitrate, Sulphate, DO and BOD values were within the permissible limits. But total alkalinities and magnesium values were exceeding the permissible limits as prescribed by Indian Standards. However, the W.Q.I. values in the present investigation were reported to be less than 75 (67.20, 68.43 and 70.37) for different season indicating that the water quality is poor and not totally safe for human consumption.
Copy the following to cite this article:
Thakor FJ, Bhoi DK, Dabhi HR, Pandya SN, Nikitaraj BC. Water Quality Index (W.Q.I.) of Pariyej Lake Dist. Kheda - Gujarat. Curr World Environ 2011;6:225-231 DOI:http://dx.doi.org/10.12944/CWE.6.2.19
Copy the following to cite this URL:
Thakor FJ, Bhoi DK, Dabhi HR, Pandya SN, Nikitaraj BC. Water Quality Index (W.Q.I.) of Pariyej Lake Dist. Kheda - Gujarat. Curr World Environ [serial online] 2011;6:225-231. Available from: http://www.cwejournal.org/?p=243
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2011-04-04 |
|---|---|
| Accepted: | 2011-05-08 |
Introduction
The use of fertilizers, pesticides and manure are main source of water pollution in this area. Water is one of the most important factor for every living organism on this planet. Water is generally used for drinking, fisheries and other domestic purposes in this area. The available fresh water to man is hardly 0.3 to 0.5% of the total water available on the earth and therefore its judicious use in imperative. Lakes are one of the important water resources used for irrigation, drinking, fisheries and flood control purposes. (Adarsh kumar et al. 2006). On the other hand, lakes also provide a habitat for invertebrates, fishes and aquatic birds. Therefore scientific study needs to review strategies for conservation and better utilization of lakes. It is with this background, the present work was undertaken between Dec. 20009 to Jan. 2010.
Water quality index (W.Q.I.) provides a single number that expresses overall water quality at a certain location and time, based on several water quality parameters. The objective of water quality index is to turn complex water quality data into information that is understandable and used by the public. A single number cannot tell the whole story of water quality parameters that are not included in the index. However, a water quality index based on some very important parameters can provide a single indicator of water quality. In general, water quality indices incorporate data from multiple water quality parameters into a mathematical equation that rates the health of a lake with number.
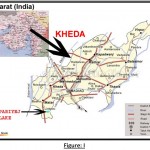 |
Figure 1: Click here to View figure |
Study Area
Pariyej lake is big in size covers an area of about 361 ha. It is situated at a distance of about 25 km from Nadiad and comes under Kheda district. It receives rain water from surrounding area and fresh water from Mahi channel. It is located in 220 32’N latitude and 720 37’E longitude. Pariyej lake is old and man made reservoir. The water is used for drinking, fisheries, agriculture and domestic purposes. The study was carried out for one year period during Dec. 2009 to Jan. 2010.
Methodology
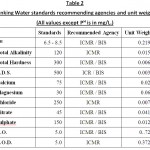
The water sample from the lake were collected at an interval of 30 days and analysed for 11 physico – chemical parameters by following the established procedures. The parameters PH and dissolved oxygen were monitored at the sampling site and other parameters like total dissolved solids, total alkalinity, total hardness, calcium, magnesium, chloride, nitrate, sulphate and biological oxygen demand were analysed in ht elaboratory as per the standard procedures of APHA (2005) and D. K. Bhoi (2004).
In this study for the calculation of water quality index eleven important parameters were chosen. The W.Q.I. has been calculated by using the standards of drinking water quality recommended by the World Health Organisation (WHO), Bureau of Indian Standards (BIS) and Indian Council for Medical Research (ICMR). The weighted arithmetic index method (Brown et. al.) has been used for the calculation of WQI of the lake. Further quality rating or sub index (qn) was calculated using the following expression.
qn=100[Vn-V10] / [Sn-V10]
Where, qn = Quality rating for the nth water quality parameter.
Vn = Estimated value of the nth parameter at a given sampling station.
Sn = Standard permissible value of the nth parameter.
V10 = Ideal value of nth parameter in a pure water.
Ideal value in most cases V10 = 0 except in certain parameters like PH and dissolved oxygen. Calculation of quality rating for PH and DO is 7.0 and 14.6 mg/L respectively.
Unit weight was calculated by a value inversely proportional to the recommended standard values Sn of the corresponding parameters.
Wn = K / Sn.
Where, Wn = Unit weight for the nth parameter.
Sn = Standard value for nth parameter.
K = Constant for proportionality.
The overall Water Quality Index (W.Q.I.) was calculated by aggregating the quality rating with the unit weight linearly.
∴ W.Q.I = ∑qnWn / Wn
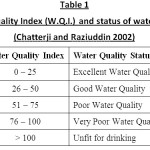 |
Table 1: Water Quality Index (W.Q.I.) |
 |
|
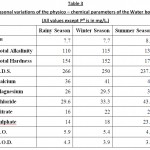 |
Table 3: Seasonal variations of the physico–chemical parameters of the Water body. |
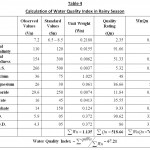 |
Table 4: Calculation of Water |
 |
Table 5: Calculation of Water |
 |
Table 6: Calculation of Water |
Discussion
Water quality index of the present lake is established from important various physico – chemical parameters in different seasons. The values of various physico – chemical parameters for calculation of water quality index are presented in Table 3. Season wise water quality index calculations are depicted in the Table 4,5 and 6. The water quality index obtained for the lake in different seasons of study period i.e., rainy season, winter season and summer season are 67.21, 68.43 and 70.37 respectively which indicate the poor quality of water (Chatterji and Raziuddin 2002). This water quality rating study clearly shows that, the status of the water body is eutropic and it is unsuitable for the human use. It is also observed that the pollution load is relatively higher during summer season when compared to the winter and rainy season. The above water quality is also supported by the following physico – chemical parameters variations observed during the different seasons of the study.
PH
PH is one of the most important factors that server as an index for the pollution. The average PH values of the lake water was 7.2 during rainy season, 7.7 during winter season and 8.0 during summer season. The PH of water was relatively high in the summer season and low in monsoon and winter season. However, when the average values for three seasons are taken into account the water body was found to be slightly alkaline. Swarnalatha and Mazasingcerao (1993) and sinha (1995). The alkaline nature of water was a characteristic throughout the study period with slight seasonal variation at the lake. In the present investigation PH values were within the ICMR standards (7.8 – 8.5)
T.D.S.
The total dissolved solids in water of Pariyej lake was266 mg/L during rainy season, 250 mg/L during winter season and 237.5 mg/L during summer season. The concentration is high during rainy season, which may be due to addition of solids from run off water, sewage and industrial effluents to the lake. Gupta and Singh (2000) also reported high concentration of TDS in the Damodar river due to mixing of sewage and industrial water.
Total Alkalinity
Alkalinity value less than 100 mg/L is desirable for domestic use. According to USPHA the maximum permissible limit is 120 mg/L. The observed average value of total alkalinity was 110 mg/L during rainy season, 115 mg/L during winter season and 136 mg/L in summer season. Total alkalinity values in our observations indicated that the water was hard. Higher values of alkalinity registered during summer might be due to the presence of excess of free CO2 product as a result of decomposition process coupled with the mixing of sewage and domestic waste. The low alkalinity during rainy season may be due to dilution. Jain et. al (1996) also reported similar finding in the study of the Halali Reservoir.
Total Hardness
The observed average total hardness value was 154 mg/L during rainy season, 162 mg/L during winter season and 170 mg/L during summer season, Higher values of hardness during summer can be attributed to low water level and high rate of evaporation of water and addition of calcium an d magnesium salts. Mohanta and Patru (2000) stated that addition of sewage, detergents and large scale human use might be the cause of elevation of hardness.
Kannan (1991) has classified water on the basis of hardness values in the following manner, 0 – 60 mg/L soft, 61 – 120 mg/L Moderately hard, 121 – 160 mg/L. hard and greater than as 180 mg/L very hard. Pariyej lake water was moderately hard but the hardness values were in permissible limits. Hardness below 300 mg/L is considered potable but beyond this limit produces gastrointestinal irritation, (ICMR, 1975).
Calcium & Magnesium
The observed average value of calcium was 36 mg/L during rainy season, 41 mg/L during winter season and 47 mg/L during summer season. The quantities of calcium in natural water depend upon the type of rocks. Small concentration of calcium is reducing corrosion in water pipes. While the observed average value of magnesium was 26 mg/L during rainy season, 29.5 mg/L during winter season and 33 mg/L during summer season. Magnesium hardness particularly associated with the sulphate ion has laxative effect on persons un accustomed to it (Khursid, 1998).
Chloride
Chloride occurs in all types of natural waters. The high concentration of chloride is considered to be an indication of pollution due to high organic waste of animal origin (Singh, 1995). Chloride value obtained in the study was 29.6 mg/L during rainy season, 33.3 mg/L during winter season and 43.5 mg/L in summer season. The chloride in Pariyej lake water was found within the acceptable limit of 250 mg/L. In natural surface water the concentration of chloride was normally low.
Nitrate
Nitrate is the most important nutrient in an ecosystem. Generally water bodies polluted by organic matter exhibit higher values of nitrate. Nitrate value obtained in the study was 16 mg/L during rainy season, 22 mg/L during winter season and 27 mg/L during summer season. In the present study water samples of all the seasons showed low concentration of nitrate well below permissible levels as per the standards.
Sulphate
Sulphate ion does not effect the taste of water if present in low concentration. The sulphate ion concentration in 14 mg/L during rainy season, 18 mg/L during winter season and 23.4 mg/L during summer season. The sulphate in Pariyej lake water was found within the acceptable limit of 150 mg/L.
DO
The average dissolved oxygen was 5.9 mg/L during rainy season, 5.4 mg/L during winter season and 4.9 mg/L during summer season. The maximum dissolved oxygen in the water of Pariyej lake was recorded in rainy season. Thereafter it started declining gradually and in summer reached the lowest concentration. This can be attributed to addition of effluents containing oxidizable organic matter and consequent biodegradation and decay of vegetation at higher temperature leading to consumption of oxygen from water.
Concentration below 5 mg/L may adversely affect the functioning and survival of biological communities and below 2 mg/L may lead to fish mortality. Water without adequate DO may be considered waste water. Presence of DO in water may be due to direct diffusion from air and photosynthetic activity of autotrophs. (Shanthi et al. 2002). The DO values obtained in the present study are slightly increased compared to ICMR standards.
BOD
BOD is the measurement of the amount of biologically oxidizable organic matter present in the waste. The increased levels of BOD indicated the nature of chemical pollution. The average BOD was 4.3 mg/L during rainy season, 3.9 mg/L during winter season and 3.6 mg/L during summer season. The BOD values obtained in the present study are within the ICMR standards.
Conclusion
Some of the samples have Total alkalinity and Magnesium values exceeding the permissible limits as prescribed by Indian standards. However, the WQI values in the present investigation are reported to be less than 75 (67.201, 68.43 and 70.37) for different season indicating that the water quality is poor and not totally safe for human consumption.
References
2. Adarsh kumar, T. A. Qureshi, Alka Parashar and R. S. Patiyal., Seasonal variation in physico-chemical characteristics of Ranjit Sagar reservoir, Jammu and Kashmir, J. Echophysiol. Occup. Hlth. 6 (2006).
3. BIS. Analysis of water and waste water. Bureau of Indian Standards, New Delhi. (1993).
4. BIS Standards of water for drinking and other purposes Bureau of Indian Standards, New Delhi. (1993).
5. Brown, R. M. N. J. McCleiland, R. A. Deininger and M. F. O. Connor. A water quality index – crossing the psychological barrier (Jankis, S. H. ed.) Proc. Int. Conf. on Water Poll. Res. Jerusalem,
6: 787-797 (1972). 6. Chandaluri Subba Rao, B. Sreenivasa Rao, A.V.L.N.S.H. Hariharan and Manjula Bharathi. Determination of Water Quality Index of some areas in Guntur District Andhra Pradesh. IJAGPT Vol (I) P. 79-86 (2010).
7. Chaterjee, C. and Razuddin, M. Determination of Water Quality Index (W.Q.I.) of a degraded river in Asanil Industrial area, Ranigunj, Burdwan, West Bengal Nature, Environment and Pollution Technology, 1(2): 181-189 (2002).
8. D.K. Bhoi, D.S. Raj, Y.M. Mehta, M. T. Machhar and M. D. Chauhan. Oriental Jaurnal chemistry 20(2): 361-364 (2004).
9. Gupta B. K. and G. Singh. Damodar river water quality status along Dugda – Sindri industrial belt of Jharia coalfield, in: Pollution and Biomonitoring of Indian Rivers, ABD Publication, JMaipur, 58 – 69 (2000).
10. ICMR Manual of standards of quality for drinking water supplies. ICMR, New Delhi. (1975).
11. Jain S. M., Meenakshi Sharma and Ramesh Thakur. Seasonal variation in Physico – chemical parameters of Halali reservoir of Vidisha district, Indian Journal of Ecobiology 8:3, 81 – 188 (1996).
12. Kannan, K., Fundamentals of Environmental Pollution. S. Chand and Company Ltd. New Delhi (1991).
13. Khursid. S, Zaheeruddin and A. Basheer. Ind. J. Env. Prot. 18(4): 246-249 (1998).
14. Mahanta H., S.S. Dhillon, K. S. Bath and G Mander. Abiotic and biotic components of a freshwater pondof Patiala (Punjab), Polln. Res. 15(3): 253-256 (1996).
15. Shanthik, P Ramaswamy and Lashman Perumalswamy. Hydrological study of Singanallur lake of Coimbatore, Nature Environment & Pollution Technology, 1(2): 97 -101 (2002).
16. Singh, J. P. and P. K. Ray., Limno Biotic Investigation of Kawar lake, Begusarai, Bihar. (Environment and Ecology) 13(2): 330-335 (1995).
17. Sinha S. K. Potability of some rural pond water at Muzaffarpur (Bihar). A note of water quality index, J. Pollution Research, 14(1):135-140 (19945).
18. Swarnalatha, N. and A. Narasingrao., Ecological investigation of two lentic environments with reference to cyanobacteria and water pollution. Indian J. Microbiol. Ecol. 3: 41-48 (1993).
19. WHO. International Standards for Drinking Water. World Health Organization, Geneva, Switzerland (1992).






