Pysico-Chemical Analysis of Bore Wells and Open Wells Drinking Water of, Kathalal Region
D. G. Shah1 * and P. M. Trivedi2
1
Department of Chemistry,
Parekh Brothers Science College,
Kapadwanj,
India
2
Department of Chemistry,
St. Xavier’s College,
Ahmedabad,
India
DOI: http://dx.doi.org/10.12944/CWE.6.2.14
Physico-chemical analysis such as temperature, PH, dissolved oxygen, total dissolved solids, chloride, total alkalinity, calcium and magnesium hardness, sulphate, phosphate nitrate of bore wells water was carried out from twenty sampling station of kathalal territory area during the May-2011 in order to assess water quality index.
Copy the following to cite this article:
Shah DG, Trivedi PM. Pysico-Chemical Analysis of Bore Wells and Open Wells Drinking Water of Kathalal Region. Curr World Environ 2011:6;287-290 DOI:http://dx.doi.org/10.12944/CWE.6.2.14
Copy the following to cite this URL:
Shah DG, Trivedi PM. Pysico-Chemical Analysis of Bore Wells and Open Wells Drinking Water of Kathalal Region. Curr World Environ [serial online] 2011;6:287-290. Available from: http://www.cwejournal.org/?p=1426
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2011-09-01 |
|---|---|
| Accepted: | 2011-10-04 |
Introduction
In continuation of our earlier analysis on bore wells water,1-3 here we report the Physico-chemical analysis of bore wells drinking water of kathalal territory. kathalal is located in kheda district of Gujarat bore wells water is generally used for Drinking and other domestic purposes in this area .The use of fertilizers and pesticides manure, lime, septic tank, refuse dump, etc, are the main sources of bore wells water pollution4 in the absence of fresh water supply, people residing in this area forced to use bore wells water for their domestic and drinking consumption. In order to assess water quality index, we have carried out the Pysico-chemical analysis of bore wells drinking water.
Experimental
In the present study bore wells water sample from twenty difference areas located in and around kathalal territory were collected in brown glass bottles with necessary precautions
Physico-Chemical analysis
All the chemicals used water of AR grade. Double distilled water was used for the preparation of reagents and solutions. The major water quality parameters considered for the examination in this study are temperature PH ,dissolved oxygen (DO)total dissolved solid(T.D.S),total alkalinity, calcium and magnesium hardness, sulphate, phosphate and nitrate contents.6 Temperature pH, dissolved oxygen (DO) total dissolved solid (T.D.S), phosphate, Nitrate values were measured by water analysis kit and manual methods. Calcium and magnesium hardness of water was estimated by complexometic titration method4,5.Chloride contents were determined volumetrically by silver nitrate titration method using potassium chromate as indicator and was calculated in terms of mg/L. sulphate contents were determined by volumetric method.5
Results and Discussion
The Physico-chemical data of the bore wells water samples collected in May-2011 are present in table -1 respectively. The results of the samples vary with different colleting places because of the different nature of soil contamination.6 All metabolic and physiological activities and life processes of aquatic organisms are generally influenced by water temperature.
Temperature
In the present study temperature ranged was kept from 29.4octo 34.3oc.
pH
In the present study pH ranged from 6.9to 8.3 which lies in the range prescribed by APHA.1 The pH value of drinking water is an important index of acidity, alkalinity and resulting value of the acidic basic interaction of a number of its mineral and organic components. pH below 6.5 starts corrosion in pipes. Toxic metals which are present in water increase the pH value of water. The tolerance pH limit is 6.5 to 8.5.
TDS
In the present study TDS ranged from 185 mg/l to 1380 mg/l. according to WHO and Indian standards.7,8 TDS value should be less than 500 mg/l for drinking water. All the sample station except sample station no 14 higher ranged as prescribed by WHO and Indian standards.7,8
D.O.
In the present study dissolved oxygen (D.O) ranged from 6.4 mg/l to 10mg/l. The minimum tolerance range is 4.0 mg/l for drinking water.
Chlorides
The chlorides contents in the samples between 28.48mg/l to 285.40 mg/l natural water contain low chloride ions. In the present study sample No.7 shows 315.75 mg/l chloride which is highest value in twenty different sampling stations. The tolerance range for chloride is 200 to 1000mg/l.7,8
Total Alkalinity
In the present study total alkalinity range was kept from 148 mg/l to 856 mg/l.
Calcium Hardness
The calcium hardness range was from 8.02 mg/l to 144.3mg/l. The tolerance range for calcium hardness is 75 to 200 mg/l. Calcium contents in all samples collected fall within the limit prescribed. Calcium is needed for the body in small quantities, though water provides only a part of total requirements.
Magnesium Hardness
Magnesium hardness ranged from 19.44 to 182.74 mg/l. The tolerance range for magnesium is 50 to 100 mg/l.
Sulphate
Sulphate ranged from 19.41 mg/l to 384.30 mg/l. The tolerance range for sulphate is 200 to 400 mg/l. The high concentration of sulphate may induce diarrhea.
Phosphate
In the present study phosphate ranged from 4.0 mg/l to 42 mg/l. The evaluated value of phosphate in the present study is much higher than the prescribed values. The higher values of phosphate are mainly due to use of fertilizers and pesticides by the people residing in this area. If phosphate is consumed in excess, phosphine gas is produce in gastro-intestinal tract on reaction with gastric juice. This could eve lead to the death of consumer.
Nitrate
In the present study nitrate ranged from 60 mg/l to 450 mg/l. The tolerance range for nitrate 20mg/l to 45 mg/l. Nitrate nitrogen is one of the major constituents of organisms along with carbon and hydrogen as amino acid, protein and organic compounds present in the bore wells water. In the present study nitrate nitrogen levels show higher values than the prescribed values. This may be due the excess use of fertilizers and pesticides in this area.
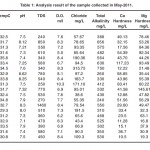 |
Table 1: Analysis result of the sample collected in May-2011 Click here to View table |
Acknowledgements
The author is also thankful to the principal of P.B.Science college of Kapadwanj for providing me to use the facilities of laboratory work.
References
1. APHA, Standard methods for the examination of water and waste water; Washington USA,1995.
2. Praharaj A.K, Mohanta B K and Manda N K, Poll Res., 2004, 23 (2), 399-402.
3. Madhavi A., Poll Res., 2005,24(2), 395-400.
4. Prajapati J. R. and Raol B. V, Poll Res., 2004,23(1), 165-168.
5. Patel K. P, Poll Res., 2003, 22(2), 241-245.
6. Mitra A and Gupta S. K J Indian Soc Soil Sci., 1999, 47, 99-105.
7. WHO, Guidelines for drinking water quality I Geneva, 1984.
8. WHO, International Standards for drinking water WHO, Geneva.1994.






