Diversity of Arbuscular Mycorrhizal Fungi in Disturbed and Undisturbed Forests of Karbi Anglong Hill District of Assam
D. Sharmah1 * and D.K. Jha1
1
Microbial Ecology Laboratory, Department of Botany,
Gauhati University,
Guwahati,
India
Corresponding author Email: dsharmah@yahoo.com
DOI: http://dx.doi.org/10.12944/CWE.6.2.07
Copy the following to cite this article:
Sharmah D, Jha DK. Diversity of Arbuscular Mycorrhizal Fungi in Disturbed and Undisturbed Forests of Karbi Anglong Hill District of Assam. Curr World Environ 2011:6;253-258 DOI:http://dx.doi.org/10.12944/CWE.6.2.07
Copy the following to cite this URL:
Sharmah D, Jha DK. Diversity of Arbuscular Mycorrhizal Fungi in Disturbed and Undisturbed Forests of Karbi Anglong Hill District of Assam. Curr World Environ [serial online] 2011;6:253-258.
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2011-06-20 |
|---|---|
| Accepted: | 2011-07-29 |
Introduction
Microbes are essential components of Earth’s biota contributing to the maintenance of Earth’s ecosystem, biosphere and biogeochemical cycling.1 Arbuscular Mycorrhizal Fungi (AMF) belonging to the phylum Glomeromycota are ubiquitous in natural ecosystem and form mutualistic symbiotic associations with majority of terrestrial plant species.2 AMF are receiving worldwide attention because of the pivotal role they play in plant community ecology and plant productivity.3 The associations are normally mutualistic, based on reciprocal exchange of resources: photosynthates from plant to fungus and soil derived limiting nutrients from fungus to plant.4 Besides, they improve plant fitness by improving tolerance to some root pathogens, water relations and formation and stability of soil aggregates.5, 6 Recent experimental work has conclusively shown that increasing AMF species richness can influence plant community structure and an increase in plant productivity and diversity.7, 8 Because of the beneficial effects on plant as well as soil health, the importance of AMF in restoration and re-establishment of fragile and degraded ecosystem is well recognized.9, 10, 11 This explains the need to enumerate and identify the indigenous AMF population which can be successfully applied in restoration practices.
Conversion of tropical forests to agricultural lands is one of the leading causes of biodiversity loss.12 The destruction of these forests is taking place at an alarming rate in tropics and the forests of Karbi Anglong Hill Disrict of Assam are also no exception to this. The major threats to the rich biodiversity of the region are expansion of agricultural activities, shifting cultivation, encroachment etc,13 The studies on the impact of these vegetation disturbances on microbial flora in general, and AMF in particular, is almost nil in this part of Indo-Burma biodiversity hotspot region. Therefore, to assess the impact of such forest destruction on microbial diversity, the present study investigated the diversity of AMF in undisturbed forests (UF), slash-and-burn fields (SBF) and monoculture forests (MF). The study also aimed to catalogue the Arbuscular Mycorrhizal (AM) fungal species so that they can be used in future for restoration and regeneration of degraded forests and maintenance of sustainable forestry.
Materials and Methods
Study Site
The study site is located in Karbi Anglong Hill District (92045’ and 93054’ East Longitude and 25045’ and 26035’ North Latitude) of Assam, India. The three sites- UF, SBF and MF are located adjacently on a hilly slope at an altitude of 232 MSL. Annual mean air temperature was 24.40C Total annual precipitation was 1052 mm, and the rainy season lied between May to September (730 mm). The soil was classified as sandy loam.
The undisturbed forest is a moist semi-evergreen forest.14 The top canopy comprised of plants like Stereospermum personatum, Duabanga sonneretiodes Ham, Terminalia chebula Retz., Tetrameles nudiflora R.Br, Amoora wallichi King., Pterospermum acerifolium Willd., Tectona grandis Linn, etc. The dominant middle storey plants were Lannea grandis A.Rich, Sterculia villosa Roxb, Dysoxylum binectariferum HK.f.et Bedd, Premna bengalensis Clarke, Mallotus philippinensis Muell. Arg, Magnolia sp, Michelia sp.
The undisturbed forest was converted to slash-burn field six months ago by clear-cutting and burning of the slashed dry biomass by local tribal peoples. Local variety of rice was cultivated as the crop after forest clearance.
Reserve forest areas subjected to Jhum or shifting cultivation are artificially regenerated by Forest Departments under jhum area rehabilitation programme to improve and restore the fragile ecosystem of the hills. Our study site MF is about 20 years old and consists of artificially regenerated fast growing species of teak (Tectona grandis Linn.).
Sampling Procedure
Sixteen soil samples from the rhizosphere region of plants were arbitrarily collected in June-July 2009, at sampling points 20 m apart on the middle line transect of each site. Each soil sample was 200 g to a depth of 15 cm. The soil was placed in sealed plastic bags and stored at 40C until analysis.
Spore Isolation and Identification of AM Fungi
One hundred grams of soil from each field sample was used for spore isolation, using wet sieving and decanting method of Gerdemann and Nicolson.15 All healthy AMF spores were counted using stereo-zoom microscope. Intact AM spores were transferred onto glass slides containing polyvinylalcohol-lactophenol with or without Melzer’s reagent and identified under a compound microscope at upto 400x magnification. AM fungi were identified using keys of Schenck and Perez16 and culture database established by the International collection of Vesicular and Mycorrhizal Fungi (http://invam.caf.wvu.edu). Permanent slides are stored at Microbial Ecology Laboratory, Dept of Botany, Gauhati University, Assam, India.
Statistical Analysis
AM fungal composition in field samples was evaluated based on spore isolation frequency (IF), density, relative abundance (RA), Shannon-Wiener index of diversity (H'), Simpson diversity index (D) and Sorenson’s similarity coefficients (Cs).
Results
AM Fungal Composition
A total of 12 AM fungal taxa were isolated from forty eight soil samples collected at three different sites. Five of these (41.6%) were identified to species level, and seven (58.3%) were identified to genus. Seven of them belonged to the genus Glomus, three to Acaulospora, one to Ambispora and one to Gigaspora. All the 12 AM fungal taxa were found in UF, whereas SBF and MF harboured only 7 and 4 taxa respectively. The Shannon-Wiener index of diversity (H') was higher in the UF (2.08) than in the SBF (1.66) or MF (1.17). The Sorenson’s similarity coefficients (Cs) of AM fungal community composition was higher between UF and SBF (0.73) than between UF and MF (0.5). Similarly, Simpson’s diversity index (D) was highest in UF (0.84), slightly lower in SBF (0.78) and lowest in MF (0.65). The three diversity indexes are tabulated in Table 1.
Isolation Frequency and Relative Abundance of AMF
Glomus was the dominant genus in all three sites followed by Acaulospora (Table 2) The three species, Glomus manihotis, Glomus sp4, and Acaulospora sp1 were common in all three sites with IF > 50%. The relative abundance of dominant genus Glomus was highest in SBF (73.3%) than in UF (65.7%) and MF (64%). Acaulospora, the second most frequented genus showed the highest relative abundance in MF (35.8%) than in UF (32.2%) and SBF (24.7%). The dominant genus Glomus manihotis has the highest relative abundance in all the three sites, and was much higher in MF (46.6%) than in SBF (32.1%) and UF (24.1%).
Spore Density of AMF
There was a significant difference in the total spore density of AM fungi among the UF (879±19.5 spores per 100g soil), SBF (174±5.3 spores per 100g soil) and MF (103±4.4 spores per 100g soil). The dominant genus Glomus had the highest spore density in UF (581±23 spores per 100g soil) than in SBF (128±7 spores per 100g soil) and MF (66±6.4 spores per 100g soil), and followed by second dominant genus Acaulospora, which had 285±43.2 spores per 100g soil in UF, 43±11.8 spores per 100g soil in SBF and 37±8.2 spores per 100g soil in MF. Spore density of four AM fungus genera isolated from 3 sites is given in Figure 1.
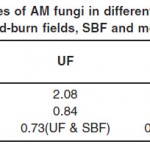 | Table 1: Diversity indices of AM fungi in different study sites (undisturbed forests, UF; slash-and-burn fields, SBF and monoculture forests, MF) Click here to View table |
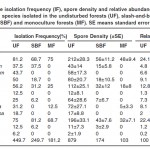 | Table 2: The isolation frequency (IF), spore density and relative abundance (RA) of AM fungal species isolated in the undisturbed forests (UF), slash-and-burn fields (SBF) and monoculture forests (MF). SE means standard error. Click here to View table |
 | Figure 1: Spore density of four AM fungus genera isolated from different study sites (UF, SBF and MF). Click here to View figure |
Discussions
Since spores are highly resistant to adverse environmental conditions,17 we used spore quantification to enumerate the diversity of AMF in disturbed and undisturbed forests. The three sites i.e. UF, SBF and MF were selected in such a way that they were located adjacently to each other on a hilly slope and with similar climatic conditions. The results of the present investigation indicated a significant influence of disturbance on total spore density of AM fungi. Spore density was highest in UF, lower in SBF, and lowest in MF. The high spore density of AM fungi in undisturbed forests with naturally higher plant species diversity suggested that disturbance affect the abundance of arbuscular mycorrhizal spores. A similar result was obtained by Su and Guo,18 who reported that the mean spore density of AMF was significantly decreased in over-grazed as compared to non-grazed plots in the Inner Mongolia steppe. Reduction in spore density due to agricultural practices has also been described by Li et al.,19 who found that the highest spore density occurred in never-cultivated field, slightly lower in old field and lowest in cultivated field in hot and arid ecosystem of Southwest China. Reduced spore density in slash-and-burn fields and monoculture forests can be explained by the fact that disturbance disrupts the soil fungi by removing above ground biomass on which these obligate symbionts depend for their carbon source and by breaking the hyphal network leading to a reduction in mycorrhizal colonization.20 Moreover, clear-cutting of forests combined with fire exposes soils to desiccation, high temperature (>500oC), and rain erosion21 and these parameters can be attributed to the lower AM fungal spore counts in slash-and-burn fields immediately after their conversion. However, other studies have documented an equal or higher AM fungal spore counts and diversity in pastures, and other deforested stands compared to adjacent natural forests.22, 23
These studies, however, attributed that the improved population may be a consequence of after land conversion which provided ample time for natural re-colonization of these sites by annual grasses and herbaceous plant species, serving as host to AMF, and thereby, is associated with higher AM fungal spore production. Although, monoculture forests site, in our study, was about 20 years old and artificially regenerated with Tectona grandis Linn., the forest floor was barren and covered with fallen twigs and leaves of the plant itself with very little or no herbaceous plant cover. Forest management factors like site preparation, shrub-clearing etc, along with high erosion rate of the sandy loam soil in sloppy tract; have resulted in the forest floor removal. Such and other reasons (like physical properties of the soil which has not been considered here) may account for the minimal AM fungal spore counts in monoculture forests.
A total of 12 AM fungal species belonging to four different genera were extracted and identified directly from the field soil samples of three study sites. All the twelve AM fungal species were isolated from undisturbed forests compared to seven from slash-and-burn fields and four from monoculture forest. The Shannon-Wiener Index of diversity was higher in undisturbed forests due to the evenness in relative abundance of the greater number of AM fungal species compared to slash-and-burn forests and monoculture forests. Sorenson’s coefficients showed a greater similarity between undisturbed forests and slash-and-burn fields due to prevalence of more number of common AM fungal species in both sites.
The prevalence of Glomus manihotis and Acaulospora sp 1 in all the three study sites along with a high IF, spore density and RA indicates that these species are more tolerant to soil disturbances. Further study is essential to establish their usefulness and role in maintaining the fragile ecosystem of this hill district.
Since in this study the samples were collected during June-July at the middle of the rainy season, and AMF being seasonal in their sporulation pattern,24 we were not sure whether all AM fungus species could be isolated, and therefore, with longer-term sampling AMF diversity in this area would definitely increase. We conclude that the forests of this hill district contained a high AM fungal diversity. The AMF diversity is significantly affected by the land use practices practiced by the people and no step has been initiated to restore this important group of microorganisms by the forest management practices.
Acknowledgements
The first author acknowledges the UGC for financial assistance in the form of Minor research Project. Assistance of Mr. Mindar Ingti and Mr. Uttam Engti during sampling is highly appreciated.
References
- Hawksworth, D.L., and Colwell, R.R. Microbial diversity: Biodiversity among microorganisms and its relevance. Biodivers. Conserv., 1: 221-6 (1992).
- Schuâler, A., Schwarzoot, D., Walker, C. A new fungal phylum, the Glomeromycota: phylogeny and evolution. Mycological Research., 105: 1413-1421 (2001).
- Klironomos, J.N., and Hart, M.M. Colonization of roots by arbuscular mycorrhizal fungi using different sources of inoculum. Mycorrhiza, 12: 181-4 (2002).
- Smith, S.E., Smith, A.F., Jakobsen, I. Functional Diversity in arbuscular mycorrhizal (AM) symbiosis: The contribution of the Mycorrhizal P uptake pathway is notcorrelated with mycorrhizal responses in growth or total P uptake. New phytol., 162: 511-524 (2004).
- Newsham, K.K., Fitter, A.H., Watkinson, A.R. Multi – functionality and biodiversity in arbuscular mycorrhizas. Trends in Ecology and Evolution, 10: 407-411 (1995).
- Smith, S.E., and Read, D.J. Mycorrhizal Symbiosis, 3rd edn. London, UK: Academic Press (2008).
- van der Heijden, M.G.A., Klironomos, J.N, Urisic, M., Moutoglis, P., Streitwolf – Engel, R., Boller, T., Weimken, A., Sander, I.R. Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability, and productivity. Nature, 396: 69-72 (1998).
- Grime, J.P., Mackey, J.M.L., Hiller, S.H., Read, D.J. Floristic diversity in a model system using experimental microcosms. Nature, 328: 420-422 (1987).
- Reeves, F.B., Wanger, D., Moorman, T., Kiel, J. The role of endomycorrhizae in revegetation practices in the semi-arid west. I. A comparison of incidence of mycorrhizae in severely disturbed versus natural environments. Am. J. Bot., 66: 6-13 (1979).
- Jasper, D. Soil Microbiology for revegetation, incorporating field inoculation with VA mycorrhizal fungi. Minerals and Energy Research Institute of Western Australia, Perth (1995).
- Wubet, T., Kottke, I., Teketay, D., Overwinkler, F. Mycorrhizal status of indigenous trees in dry Afromontane forests of Ethiopia. For. Ecol. Manag., 179: 387-399 (2003).
- Richter, B.S., Tiller, R.L., Stutz, J.C. Assessment of arbuscular mycorrhizal fungal propagules and colonization from abandoned agricultural fields and semi-arid grasslands in riparian flood-plains. Appl Soil Ecol., 20: 227-238 (2002).
- Yumnam, J,Y. Rich biodiversity of North East India needs conservation. Current Science, 95: 297 (2008).
- Rao, S.S. Working plan of Forest West Division Karbi Anglong. Forest Dept. Publication, Karbi Anglong, Assam (2004).
- Gerdemann, J.W., and Nicolson, T.H. Spores of arbuscular mycorrhizal Endogone extracted from soil by wet sieving and decanting. Trans. Br. Mycol. SOC., 46: 235-244 (1963).
- Schenck, N.C., Perez, Y. (Eds.). Manual for identification of VA mycorrhizal fungi. Synergistic, Gainesville, Fl. (1990).
- Abbot, L.K., and Robson, A.D. Factors influencing the occurrence of vesiculararbuscular mycorrhizas. Agric Ecosys Environ., 35: 121-150 (1991).
- Su, Y.Y., and Guo, L.D. Arbuscular mycorrhizal fungi in non-grazed, restored and over-grazed grassland in the Inner Mongolia Steppe. Mycorrhiza, 17: 689-693 (2007).
- Li, L.F., Li, T., Zhao, Z.W. Differences of arbuscular mycorrhizal fungal diversity and community between a cultivated land and an old field and a never cultivated field in a hot and arid ecosystem of South West China. Mycorrhiza, 17: 655-665 (2007).
- Jasper, D.A., Abbot, L.K., Robson, A.D. The survival of infective Hyphae of vesicular arbuscular mycorrhizal fungi in dry soil – an interaction with sporulation. New Phytol., 124: 473-479 (1993).
- Aguilar-Fernandez, M., Jaramillo, V.J., Varela-Fregoso, L., Gavito, M.E. Short-term consequences of slash-and-burn practices on the arbuscular mycorrhizal fungi of a tropical dry forest. Mycorrhiza, DOI 10.1007/ s00572-009-029-2 (2009).
- Picone, C. Diversity and abundance of arbuscular mycorrhizal fungus spores in Tropical Forest and Pasture. Biotropica, 32: 734-750 (2000).
- Zhang, Y., Guo, L.-D, Liu, R.-J. Survey of arbuscular mycorrhizal fungi in deforested and natural forest land in the subtropical region of Dujiangyan, China. Plant and Soil, 261: 257-263 (2004).
- Guadarrama, P., Alveraz-Sanchez, F.J. Abundance of arbuscular mycorrhizal fungi spores in different environments in a tropical rain forest, Veracruz, Mexico. Mycorrhiza, 8: 267-270 (1999).






