Groundwater Quality in Beed District of Maharashtra During Summer Season
A.V. Gaikwad1 and S.R. Mirgane2
1
Department of Chemistry,
V.M. Kaij Dist-bee,
India
2
J.E.S.C. Jalna Dist.,
Jalna,
India
DOI: http://dx.doi.org/10.12944/CWE.6.1.18
A systematic physico – chemical study of ground water in 16 different localities in Beed district of Maharashtra has been taken up to evaluate its suitability for drinking purpose in the year 2008-09. The physic–chemical parameters are such as pH, EC, TDS, TH, TA, Cl-, Ca++, Mg++, K+, Na+ of ground water were studied. In present study water samples were collected monthly for four months during summer from sixteen selected ground water sources i.e. Bore wells. The values of physcio – chemical parameters are compared with standard values suggested by WHO. It is observed that values of TDS, TH, TA,Ca++, Mg++ and Na+ have high values than the permissible limit prescribed by WHO.
Copy the following to cite this article:
Gaikwad A.V, Mirgane S.R. Groundwater Quality in Beed District of Maharashtra During Summer Season. Curr World Environ 2011:6(1);131-134 DOI:http://dx.doi.org/10.12944/CWE.6.1.18
Copy the following to cite this URL:
Gaikwad A.V, Mirgane S.R. Groundwater Quality in Beed District of Maharashtra During Summer Season. Curr World Environ 2011:6(1);131-134. Available from: http://www.cwejournal.org/?p=1299
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2010-01-03 |
|---|---|
| Accepted: | 2010-03-10 |
Introduction
Water covers about 70% 0f the earths surface. Although water on earths surface is abundant, 97% water is salty, 2% is solid water and only 1% is available as a fresh water in which ground water accounts for 98%. Water has great importance in the life of living organisms. Economy and health of nation depends upon the quality and quantity of water. Generally urban and rural area peoples are using their own Bore–wells water for drinking and domestic purposes, because related authorities are unable to meet the ever increasing demand of potable water (V.K.Garh et al 2004)
But various studies carried out past have shown that ground water is also polluted due to dumping of waste, sewage disposal, industrial effluent disposal and septic tanks.
Beed district is one such urban and rural area were 90% population is using Bore well water for drinking and domestic purposes.
The physic – chemical parameters of ground water from Beed district were studied.The samples were as follows:
| S1 | Vasant College Kaij. |
| S2 | Shukrawar peth Kaij. |
| S3 | Adas common Bore well |
| S4 | Dhande galli Beed |
| S5 | Prashant nagar Ambajogai |
| S6 | Adas road Dharur |
| S7 | Police Colony Parali |
| S8 | Near Vaidyanath Mandir Parli |
| S9 | Near Mauli Mandir Chakarwadi. |
| S10 | Shivaji Chouk Telgaon |
| S11 | Chaousala village, Beed |
| S12 | Near Bus stand Georai |
| S13 | Wida village Kaij |
| S14 | Moha village Majalgaon |
| S15 | Khadakpura Ambajojai |
| S16 | Sugarfactory Ambajogai |
These samples were accessed for water quality during summer season. The results obtained are compared with standard data prescribed by WHO.
Methods
The samples were collected in middle of each month and parameters were measured using standard methods of analysis.
The pH of samples was measured with the help of digital pH – meter ELICO – 120 and conductivity was measured by digital conduct meter ELICO-667. TDS was measured with the help of digital TDS meter. Sodium and Potassium were measured with the help of ELICO-CL-220 Flame photometer. The remaining parameters were determined as usual.
Results and discussion
The physico – chemical parameters of different samples in February, March, April and May months are given in table no. 1, 2, 3 and 4.
The standard values prescribed by WHO for pH, EC, TDS, TA, Cl-, Ca++, Mg++, Na+, and K+are 7.0 - 8.5, 750-2250 µs, 500 ppm, 200 ppm , 200 ppm, 75 ppm, 30 ppm, 20 ppm and 12 ppm respectively.
Almost all the samples in February show acidic pH which is not in the permissible limit of standard data prescribed by WHO except sample S11. In March 50% samples show alkaline while 50% are slightly acidic in nature. In April and May all samples are alkaline in nature. Electrical conductivity of all samples in summer season was within permissible limit of WHO. All samples studied during summer season has higher values of TDS, these ranges from 190 ppm to 2800 ppm. The total hardness in the studied samples varies from 70 ppm to 1290 ppm. In summer total hardness increases from February to May. Total alkalinity of all the samples in summer is above the permissible limit prescribed by WHO. It varies from 251 ppm to 700 ppm . All samples show Cl- within permissible limit except S2, S3 and S10. These are having high concentration prescribed by WHO. Cl- concentration increases from February to May in summer. Ca++ content is higher in all samples studied except few samples in different months . All samples studied during summer show higher concentration of Mg++ than permissible limit prescribed WHO.
Potassium and Sodium are naturally occurring elements in ground water. Sodium is with high concentration but Potassium is within permissible limit given by WHO. The concentration of Sodium in the studied samples varied from 58 ppm to 95 ppm. The concentration of Potassium varied from 3.60 ppm to 60.20 ppm. Sample S12 and S13 have higher values in February while sample S16 have higher values in May.
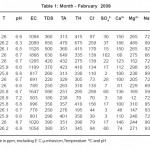 |
Table 1: Month - February 2009 Click here to View table |
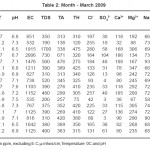 |
Table 2: Month - March 2009 Click here to View table |
 |
Table 3: Month - April 2009 Click here to View table |
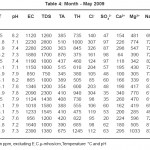 |
Table 4: Month - May 2009 Click here to View table |
Conclusion
Finally above results show that ground waters of all samples during summer also show higher values of 90 % parameters of common Bore- wells. This is because of large depth of Bore-wells. Variation in nature of rock , nature of Earth crust etc. Solid waste material deposition, improper drainage systems also changes the nature of ground water. Hence these are chemically unfit for drinking purposes and should not be used without pretreatment .
References
- APHA 1995:Standard methods for examination of water waste 19th edition .
- Trivedy R.K. and Goel P.K., Chemical and 4. Biological methods for water pollution studies (1984).
- N. Manivaskam, Physico – chemical examination of water , sewage and industrial effluents (2005).
- V.K. Garg et al., Hydrochemistry of underground water in southern zone of Hisar City Poll. Res. 23(3): 461 (2004). Enviromedia.






