Assessment of Ground Water Quality and its Impact in and around Mangalam near Tirupathi, Andhra Pradesh, India
G. Dilli Rani1 , M. Suman1 , C. Narasimha Rao1 , P. Reddi Rani1 , V. G. Prashanth2 , R. Prathibha2 and P. Venkateswarlu1
DOI: http://dx.doi.org/10.12944/CWE.6.1.30
Ground water quality and its impact on human health in and around Mangalam, near Tirupathi, India was assessed. Water samples were collected from 8 different areas in and around Mangalam and analyzed for physicochemical parameters such as pH, electrical conductivity, total dissolved solids, total hardness, calcium, chlorides, sulphates, nitrates and dissolved oxygen. The found values of physicochemical parameters were compared with the World Health Organisation water quality standards. Based on the analysis, it was found that ground water of some of the areas was polluted and not suitable for drinking purpose. Thus the ground water of the area needs purification before drinking.
Copy the following to cite this article:
Rani G. D, Suman M, Rao C.N, Rani P. R, Prashanth V. G, Prathibha R,Venkateswarlu P. Assessment of Ground Water Quality and its Impact in and around Mangalam near Tirupathi, Andhra Pradesh, India. Curr World Environ 2011:6(1);191-193 DOI:http://dx.doi.org/10.12944/CWE.6.1.30
Copy the following to cite this URL:
Rani G. D, Suman M, Rao C.N, Rani P. R, Prashanth V. G, Prathibha R,Venkateswarlu P. Assessment of Ground Water Quality and its Impact in and around Mangalam near Tirupathi, Andhra Pradesh, India. Curr World Environ 2011:6(1);191-193. Available from: http://cwejournal.org?p=332/
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2011-01-06 |
|---|---|
| Accepted: | 2011-02-26 |
Introduction
Water is the most vital source and one of the precious natural resources of this planet. Due to industrialization and urbanization ground water quality is adversely affected. According to WHO, nearly 80% of all the diseases in human beings are caused by water. Many of the people of Mangalam, near Tirupathi, Andhra Pradesh, India are affected by pollution of drinking water. In the present study an attempt was made to assess the quality of ground water and its suitability for drinking purpose.
Experimental
Water Samples and Chemicals
For the analysis of physicochemical parameters, 8 areas were selected located in and around Mangalam, near Tirupathi, India. The ground water samples were collected in clean and dry polythene bottles from bore wells after running them for 10 minutes Each sample in the polythene bottle was filtered using whatmann no.42 filter paper and stored. The water samples collected were analyzed within 5 hours after collection. The samples were collected during summer season because the mineral content in water are likely to reach the maximum. Samples were collected from shallow wells because the shallow wells are well oxygenated than deeper wells.
Methodology
Electrical conductivity values were measured using Elico CM 180 Conductivity Bridge. Total alkalinity was evaluated by titration with standard 0.1M HCl using methyl orange and phenolphthalein as indicators.1 Standard procedures2 involving spectrophotometry, flame photometry and volumetry were used for the determination of hardness, total dissolved solids (TDS), sulphate, chloride, nitrate, calcium, magnesium and iron. All the chemicals used were of AR grade.
Results and Discussion
The results obtained for the analysis of physicochemical parameters were presented in table l and the results were compared with limits prescribed by WHO.
The temperature which is responsible for different physical and biological processes depends on the type of water. The pH of the water samples analyzed were within the desirable limit of 6.7 – 7.6 given by WHO and most of the samples were slightly alkaline in nature. Alkalinity in water is due to dissolved carbon dioxide, carbonate and bicarbonates. The alkalinity varies from 250 to 463. Higher alkalinity gives unpleasant taste to water. The increased values of alkalinity in the studied area are due to the action of the carbonates on the basic material of the soil.
Electrical conductivity of water is direct function of its total dissolved salts.3 Hence it is an index to represent the total concentration of soluble salts in water.4 Most of the inorganic substances present in water are in ionized form and causes electrical conductivity. The electrical conductivity ranges from 596 to 1800 in the studied area. Electrical conductivity of water is an index to represent the total concentration of soluble salts. Hence it is is considered to be an indication5 and it is a direct function3 of the total dissolved salt content in water.4 The permissible total dissolved salts for drinking water is 500 mg/L. High values of TDS in ground water are generally not harmful to human beings but high concentrations of these may affect persons who are suffering from kidney and heart diseases.6
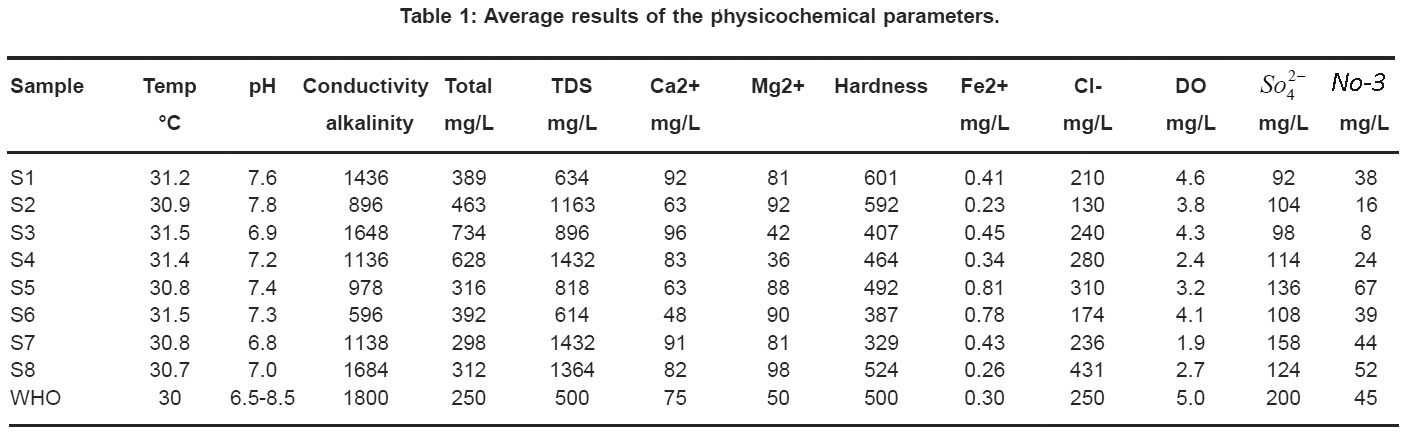 |
Table 1: Average Results of the Physicochemical Parameters. Click here to view table |
Calcium is fifth abundant element. It usually comes from the leaching of rocks. It plays an important role in the formation of bones. In this study calcium concentration of water samples ranges from 48 to 96 mg/L. If concentration of calcium exceeds, it causes gastrointestinal diseases and stone formations. Some of these values exceed the permissible limit proposed by WHO (75 mg/L). In ground water, generally magnesium content will be less than calcium content. If the concentration of magnesium in drinking water is more than the permissible limit, it causes unpleasant taste to the water. In many of the studied areas, drinking water contains more amount of magnesium than WHO standard (50 mg/L). Hardness is due to the presence of chlorides and sulphates of calcium and magnesium in water. Total hardness of water is the measure of the capacity of water to precipitate soap and is usually expressed as the equivalent of CaCO3 concentrations. Excess hardness in water leads to heart diseases and kidney stone formation.7 In some of the studied areas total hardness exceeds WHO standards (500 mg/L).
Iron is an important essential element to human body which is present in hemoglobin and myoglobin. When iron concentration exceeds permissible limit in drinking water it gives stringent taste to water. According to WHO standards, limit of iron concentration in drinking water is 0.3 mg/L exceeding which causes toxicity.
Oxygen is a regulator of metabolic processes of plants and animals. The DO level in drinking water of the studied area is low when compared to WHO standard (5.0). This depletion of oxygen level is due to high amount of organic wastes.
In those regions where the temperature is high and rainfall is less, the concentration of chlorine in ground waters is high. Soil porosity and permeability also has a key role in building up the chloride concentration.8 At concentrations above WHO standard (250 mg/L), drinking water acquires salty taste which is objectionable. The excess concentration of chloride in ground water is due to presence of soluble chloride from rocks. High amounts of sulphate cause laxative effect to the children in hot weather climates.9 In the studied area, sulphate concentration in drinking water is below the WHO standard (200 mg/L). Excessive concentration of nitrate in drinking water in considered hazardous for infants causing metheglobinaemia.10 Some of the studied regions have high concentration of nitrates than WHO standard (45 mg/L) which is due to over application of fertilizers and improper manure management practice.
Conclusions
Based on the results obtained for physicochemical analysis of ground water samples collected from different locations of Mangalam, it can be concluded that in some samples water quality parameters (Total alkalinity, pH, hardness, TDS, sulphate, chloride, nitrate, calcium, magnesium and iron) were beyond the permissible limit prescribed by WHO. Hence, drinking water pollution should be controlled by the proper environment management plan. Ground water of this area should be pretreated to make suitable for drinking and to maintain proper health conditions of people living in this area.
References
-
APHA. Standard methods for the examination of water and waste water (19th ed) Washington, DC: Public Health Association (1996).
-
Nagarajan S, Swaminathan M and Sabarathinam PL, Poll. Res., 12(4): 245 (1993).
-
Harilal CC, Harshim A, Arun PR and Baj S, J. Ecology, Environment and Conservation, 10(2): 187-192 (2004).
-
Purandara BK, Varadarajana N and Jayashree K, Poll. Res., 22(2): 189 (2003).
-
Hem JD, 1985, “Study and Interpretation of the chemical characteristics of natural water. U.S. Geol. Surv. Water Supply Paper 2254, PP 1-263.
-
Gupta S, Kumar A, Ojha AK and Singh G, J. Environmental science and Engineering, 46(1): 74-78 (2004).
-
Lalitha S, Barani AV, Indian J. Environ protect, 24(2): 925 (2004).
-
D.K. Chanda, Hydrology J, 7(5): 431-439 (1999).
-
Gupta and Suruchi, Asian J Chem,, 13(3): 16 (2001).
-
Julio AC, Alvaro A, Environment 32: 831-849 (2006).






