Equilibrium sorption studies for Cu2+ and Pb2+metal ions on three different biomasses
Uma M. K. Nagpal1 * and Hassan Rezaei2
1
Department of Geology,
University of Pune,
Pune,
India
2
Department of Environmental Sciences,
University of Pune,
Pune,
India
DOI: http://dx.doi.org/10.12944/CWE.5.2.04
Presence of heavy metals in the aquatic system is posing serious problems. The aim of this study was to utilize the locally available agricultural waste materials for scavenging these heavy metals. In the present study, the biomass generated from the dried leaves of Terminalia Catappa, Dalbergia latifolia and Ficus benghalensis was used for evaluating the biosorption characteristics of Pb and Cu ions in aqueous solutions. Batch adsorption experiments were performed on these leaves and it was found that the amount of metal ions adsorbed increased with the increase in the initial metal ion concentration. The equilibrium sorption capacity for 800 mg l-1 metal solution was 77.55, 59.35 and 19.35 mg g-1 respectively for these three leaves. Out of the two isotherms tried Langmuir gave the best fit with r2 values ranging from 0.96 to 0.98. Terminalia Catappa leaves were found to be best sorbent than Dalbergia latifolia followed by Ficus benghalensis leaves which showed least sorption. From the leaf composition from XRF, Ion exchange also could be one of the options for studying the mechanism of adsorption of these cations on these leaves. Comparing with other similar studies these were found to be the excellent adsorbents and can be successfully used by Industries for heavy metal removal
Copy the following to cite this article:
Nagpal M. K. U, Rezaei H. Equilibrium sorption studies for Cu2+ and Pb2+metal ions on three different biomasses. Curr World Environ 2010;5(2):243-251 DOI:http://dx.doi.org/10.12944/CWE.5.2.04
Copy the following to cite this URL:
Nagpal M. K. U, Rezaei H. Equilibrium sorption studies for Cu2+ and Pb2+metal ions on three different biomasses. Curr World Environ 2010;5(2):243-251. Available from: http://www.cwejournal.org/?p=1185
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2010-10-13 |
|---|---|
| Accepted: | 2010-11-20 |
Introduction
Water gets polluted from leaching of ore deposits and from anthropogenic sources which include mainly industrial effluents and solid waste disposal. Industrial development in the recent past has substantially increased the level of heavy metals.
Removal of heavy metals from aqueous solution by using inactive and dead biomass is an alternative technology for removing toxic heavy metals which cause life-threatening illnesses and pose environmental disposal problem due to their non degradable and persistence nature (Ahluwalia Goyal, 2006). There is a need to treat the industrial wastewaters before being released into the environment.
Different treatment techniques such as ion-exchange, membrane filtration, oxidation-reduction, chemical precipitation, adsorption, reverse osmosis and evaporative recovery are cost intensive, for separation of heavy metals, have been developed for effluents laden with heavy metals (Chong and Volesky, 1995; Elouear et al., 2009). The treatment of metal-bearing effluents requires more effective techniques with lower costs than the conventional ones.
Low-cost adsorbents have been evaluated for the removal of heavy metals from aqueous solutions
[Chaiyasith et al., 2006, Patterson J W, 1985; Aksu et al., 1991] because of the following:
Plants have a natural tendency to sequester heavy metals because of the presence of of an elaborate network of capillary tubes which have an excellent sorptive matrix
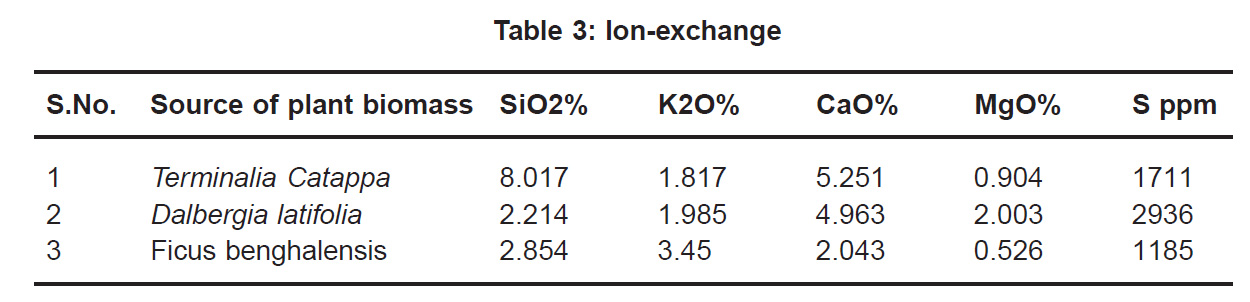
Also the cell walls of these biomasses have varied composition of proteins, carboxylic acids and phenolic compounds (M.Friedman, 1972) which allow ion exchange.
Leaf litter creates significant disposal problem and if it can be used for cleaning polluted water than there can be double benefit where the disposal problem is solved and waste becomes a useful and inexpensive sorbent for water cleaning.
No nutrients are required to keep them alive and 5.There is a possibility of metal recovery.Forest residues are low cost, renewable, and widely available making them a logical choice to remove metal ions from contaminated waters like rubber leaf powder (Hanafiah et al., 2006), Lalang leaf (Hanafiah et al., 2007) etc many low –cost adsorbents have been used to study their adsorption capacities. Saraca indica leaf powder could remove 95.37% of Pb at the pH 6.5 (Goyal et al., 2008), Mahvi et al. (2008) reported that cadmium uptake was 85% and 92% for both Ulmus leaves and their ash, respectively. The leaf biomass of Cercis siliquastrum L. eliminated Pb(II), Cu(II) and Ni(II) from waste water (Salehi*et al.,2008), ficus religiosa leaves were used for treatment of lead and chromium waste (Qaiser* et al., 2007) , S. cumini L. was chosen as a biosorbent for Lead (King et al., 2007)
From the eco-toxicological point of view Pb, Cd, Cu etc. are the dangerous metals, therefore; removal of these heavy metals from wastewaters is of prime importance.
Since Lead and Copper are widely used in Industries Pb can cause chronic toxicity and can damage the nervous system, kidneys, and reproductive system, liver and brain (Hepple, 1972). It is used in industries like storage-battery manufacture, pigments, leaded glass, photographic materials, matches and explosives, printing, pigment manufacturing, petrochemicals, fuel combustion and photographic materials (Carson et al., 1986; Raji and Anirudhan, 1997) and Cu which is relatively less toxic, however, higher concentrations are equally toxic and is mainly employed in electric goods industry, hydrometallurgy, tire manufacturing, etc
In the present study, the biomass generated from the dried leaves of Terminalia Catappa, Dalbergia latifolia and Ficus benghalensis, which are locally available plants, is used for evaluating the biosorption characteristics of Pb and Cu ions in aqueous solutions. Sorption isotherms are used to model the adsorption equilibrium.
Terminalia Catappa also called Badam has obovate leaves with smooth grey bark. Now days it is used as an ornamental tree also apart from its usage as almond fruit bearing tree. Its leaves are big in size and were collected from around the Uttam Nagar area, near NDA, Pune.
Dalbergia latifolia is a large genus of small to medium-size trees and shrubs. It is a tall,deciduous tree. It gives premium quality timber which is rich in aromatic oils. Its leaves were collected from Pune University campus area.
Banyan is found throughout the year and is very common. The leaves of banyan tree have a beautiful heart shape which taper to a needle point. When the leaves first appear their color is red-pinkish but then turn deep green and grows to about 12 to 18 cm long. The banyan tree (Ficus benghalensis), also called Indian fig,is evergreen and is supported with copious aerial roots. The bark surface is smooth and grey. Its leaves were collected from NDA area.
To know if these three biomasses are good adsorbents of Pb and Cu heavy metals, they were tested for variation with time, pH, biomass concentration and initial metal ion concentration. Composition of leaves was found with the help of ED XRF.
Material and Methods
The leaves of Terminalia Catappa, Dalbergia latifolia and Ficus benghalensis were kept in oven at 333–343 K for 24 h in an air oven. The dried leaves were then converted into fine powder by grinding in a mechanical grinder. The powder was than sieved through 10 mesh sieve and preserved as adsorbents in glass bottles for further experiments. For all experiments, the stock solutions (1000 ppm) of Cu (II) and Pb (II) were prepared in distilled water.
The three biomasses were analyzed by XRF for their chemical composition using model
AMETEK (Material Analysis Division). The 4 g. of the ground and dried samples were taken in cups for XRF determination by powder method.
To study the variation with contact time, the metal removal was tested in batches at different time intervals and the residual metal ion concentration of supernatant was determined using Varian Spectrophotometer, Model Spectra AA220.
To study the effect of pH, the pH of the metal solution was varied between 2 to 6, keeping the concentration of the biomass and contact time constant.
0.5g to 2.0g of the biomass was treated with 400 mg l-1 metal ions keeping all other variables constant. All the three biomasses were treated with varying initial metal ion concentration ranging between 50 to 800 ppm.
Adsorption isotherms
The biosorption capacities (qeq) at equilibrium were calculated as follows:
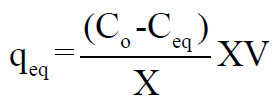
where C0 was the initial metal ion concentration (mg 1-1); Ceq the metal ion concentration at equilibrium (mg 1-1); V the volume of solution used in litres ; X was dry leaves powder weight(g).
The Langmuir isotherm model in linearized form is given as where bm is a coefficient related to the affinity between the sorbent and sorbate, and qm is the maximum sorbate uptake under the given condition.
The Freundlich isotherm in linearized form was applied as follows
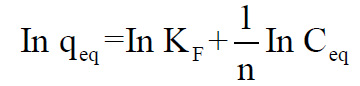
where qeq and Ceq are the biosorption capacity of the biosorbent at equilibrium and the equilibrium metal ion concentration, respectively. KF and n are adsorption isotherm parameters.
Results and Discussion
Concentration on Biosorption
It was found that the Cu and Pb metal uptake was very fast and it reached equilibrium in the first 15 minutes only which is in agreement with results of (Ajmal et al., 2006) for Cd (II) adsorption on parthenium and the weed adsorbed it in 20 min. It is in conformation with (Bhattacharyya and Gupta, 2006) who inferred that this initial high uptake is due to the bare surface after which the adsorption is gradual as the available sites decrease.
 |
 |
 |
 |
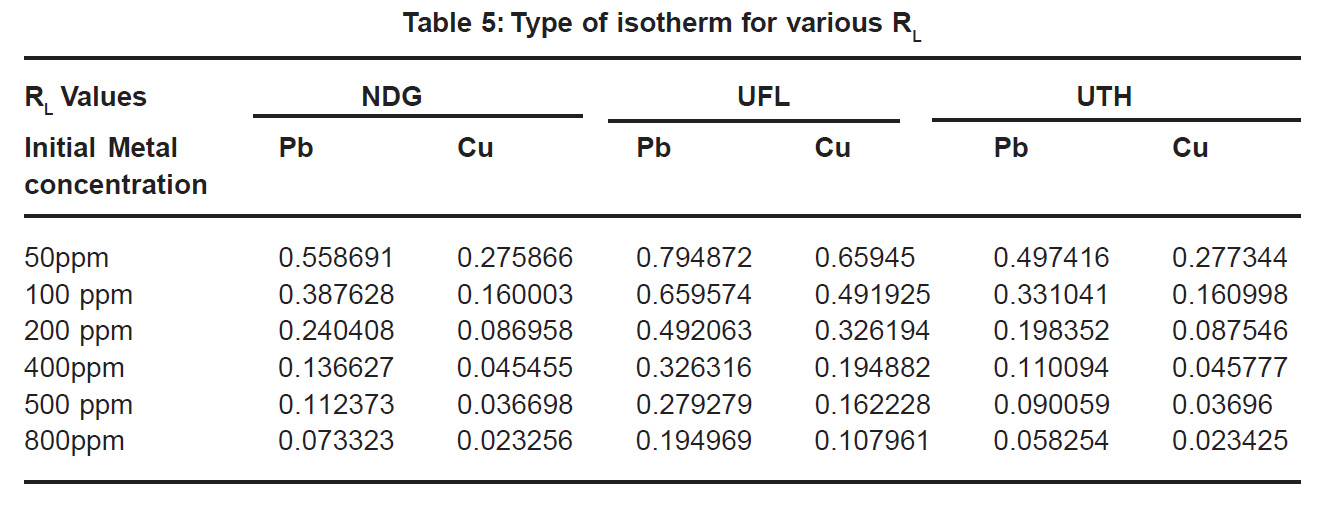 |
Table 5: Type of isotherm for various RL
Click here to view table |
From (Fig. 1 & 2) it was observed that adsorption maxima reached at pH 3 only and the adsorption values remain stable after pH 3. For further experiments the pH value was maintained at 4 throughout because at lower pH the sites on the cell wall remain protonated and don’t allow the approach of cations whereas at higher pH deprotonation takes place and the sites become negatively charged attracting the cations with different concentrations of the biomasses, it was found that lower the initial concentration of the biomass, higher was the sorption (Fig.3 & 4 ) because of the simple reason that the mass transfer is rapid at lower biomass concentrations Similar trends were observed in the sorption of cations on the waste beer yeast by (Hana et al., 2006).
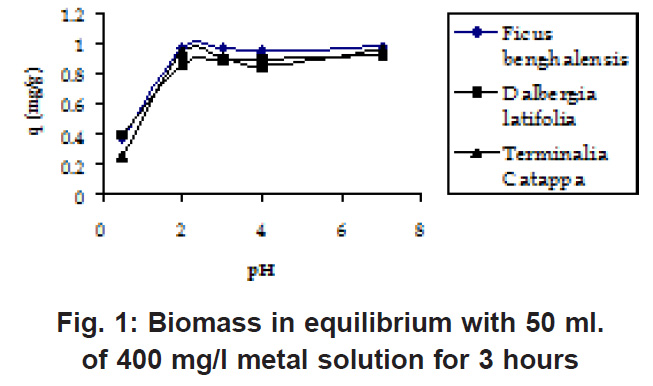 |
Figure 1: Biomass in equilibrium with 50 ml. of 400 mg/l metal solution for 3 hours Click here to view figure |
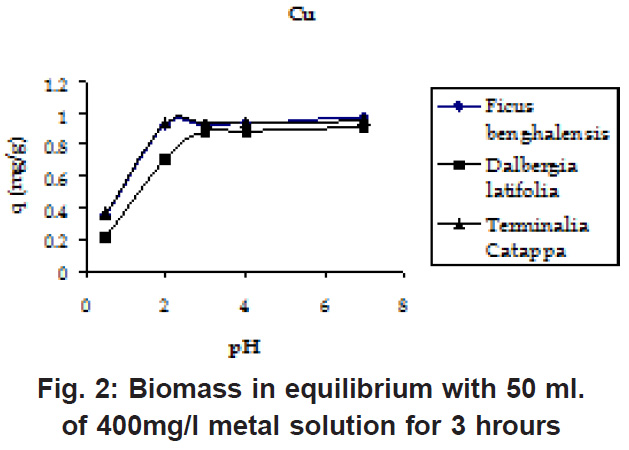 |
Figure 2: Biomass in equilibrium with 50 ml. of 400mg/l metal solution for 3 hrours Click here to view figure |
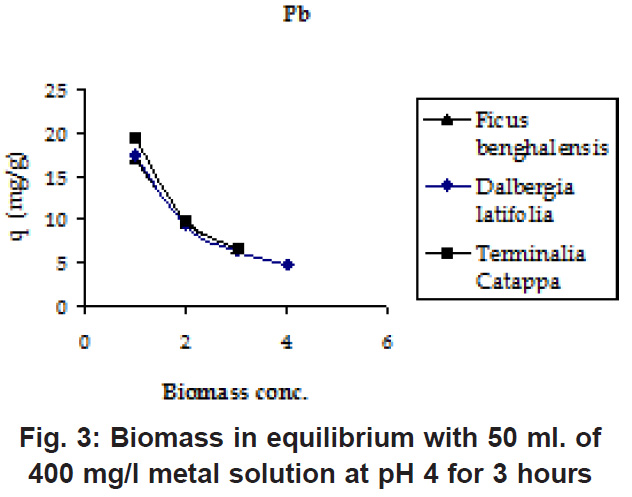 |
Figure 3: Biomass in equilibrium with 50 ml. of 400 mg/l metal solution at pH 4 for 3 hours Click here to view figure |
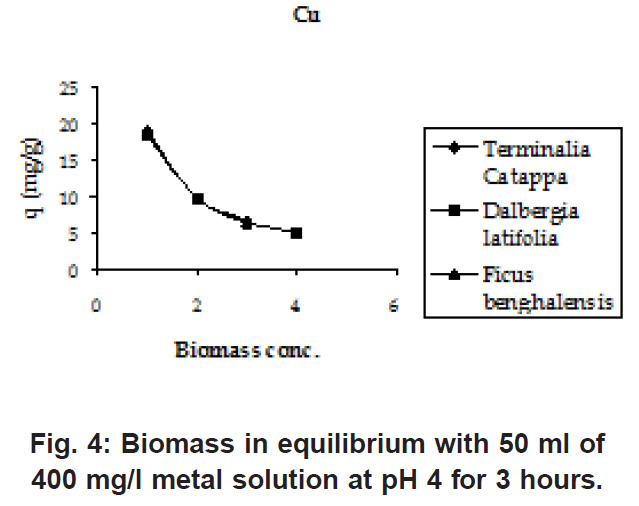 |
Figure 4: Biomass in equilibrium with 50 ml of 400 mg/l metal solution at pH 4 for 3 hours Click here to view figure |
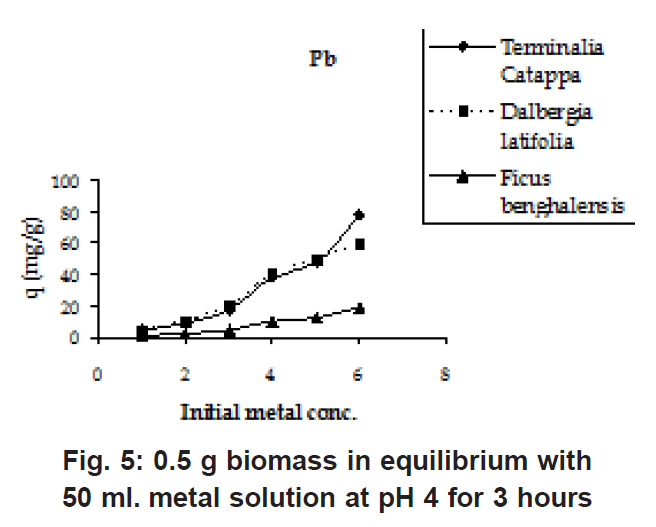 |
Figure 5: 0.5 g biomass in equilibrium with 50 ml. metal solution at pH 4 for 3 hours Click here to view figure |
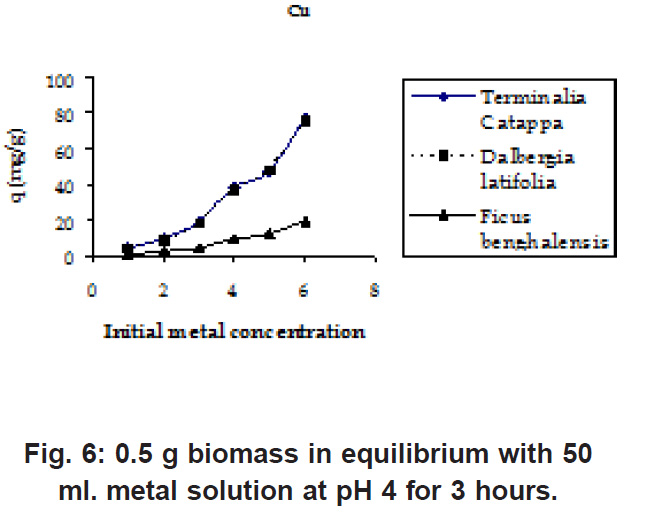 |
Figure 6: 0.5 g biomass in equilibrium with 50 ml. metal solution at pH 4 for 3 hours Click here to view figure |
Effect of initial metal ion concentration on biosorption
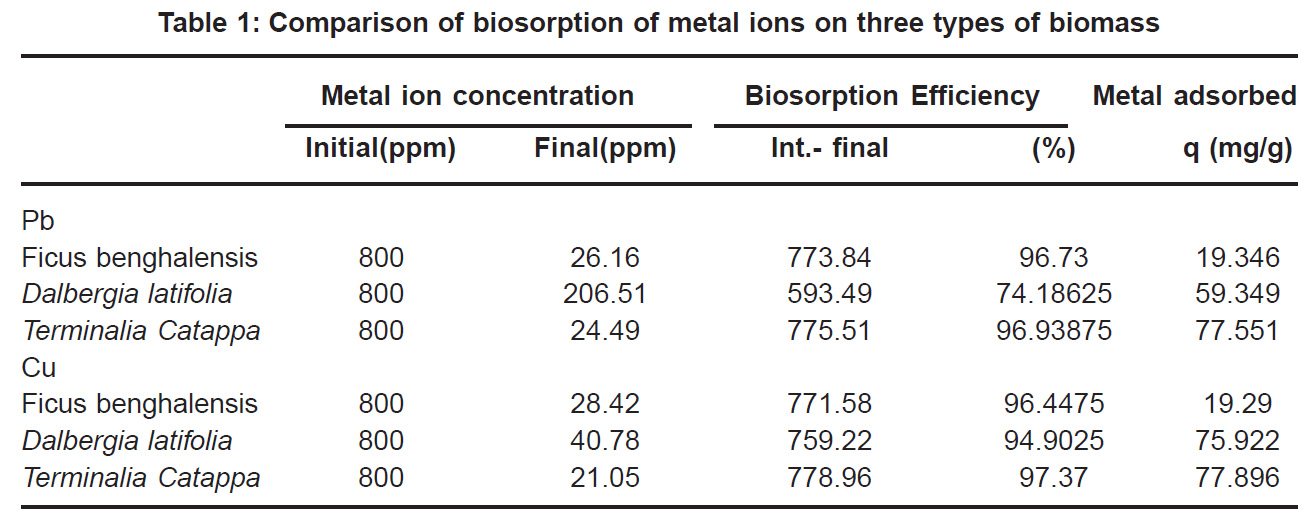
The initial Pb and Cu metal ion concentration was in the range of 50 to 800 mg/l and there is a constant increase in the uptake of the heavy metal ions as seen in the Fig. 5 & 6. Biosorption values (mg/g) were highest for Terminalia Catappa (77.896 for Cu and 77.551 for Pb) followed by Dalbergia latifolia (75.922 for Cu and 59.349 for Pb) (Table 1).The reason for this preference is that the size of the Cu metal ion which is smaller than Pb ion. Ficus benghalensis showed the lowest biosorption values (19.29 for Cu and 19.346 for Pb).
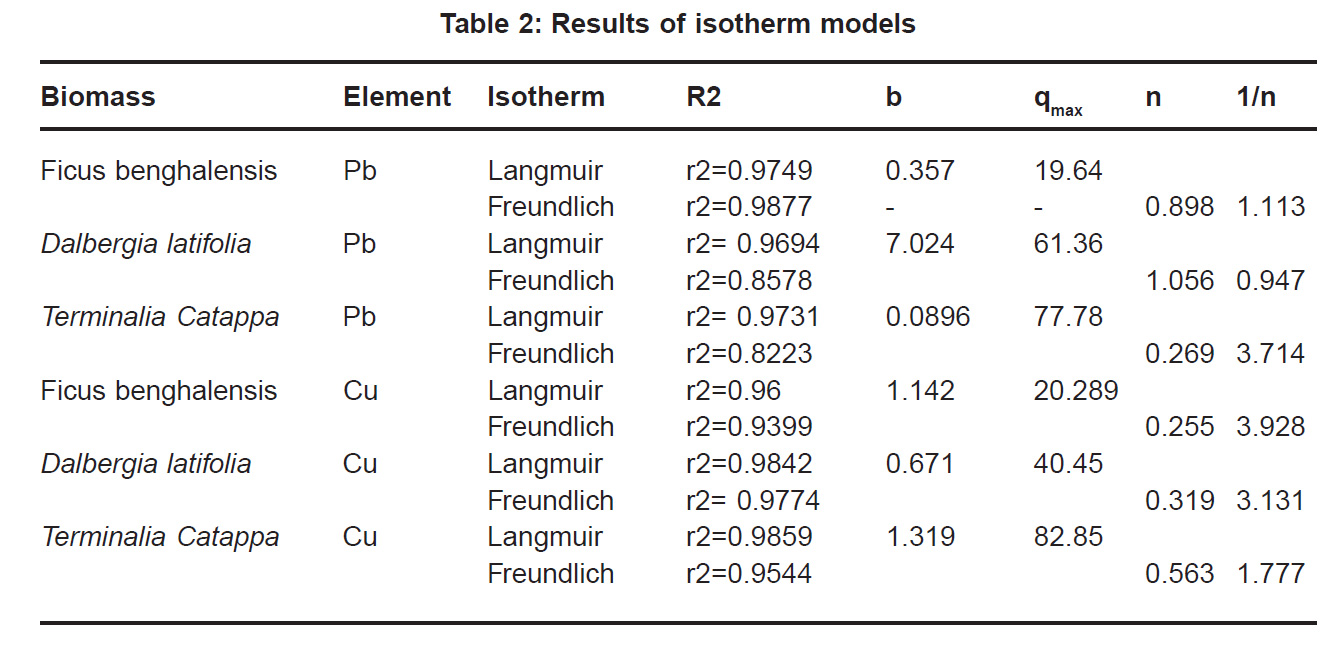
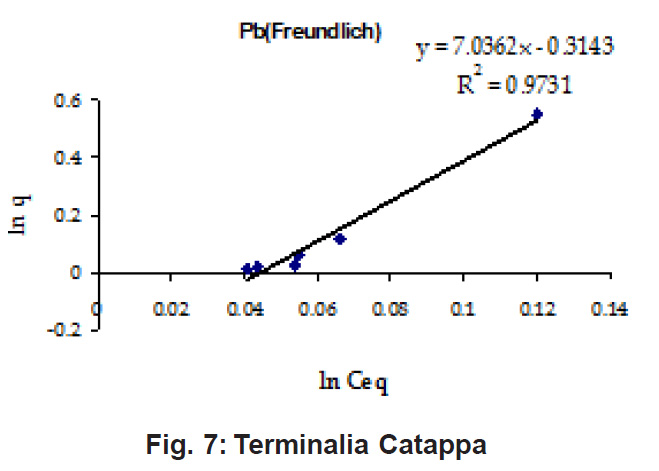
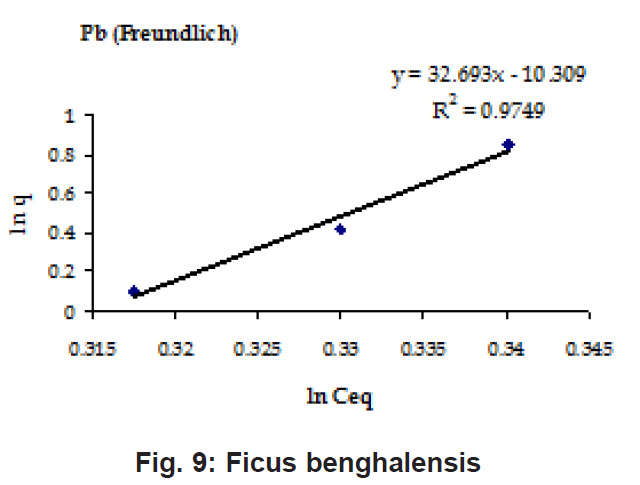
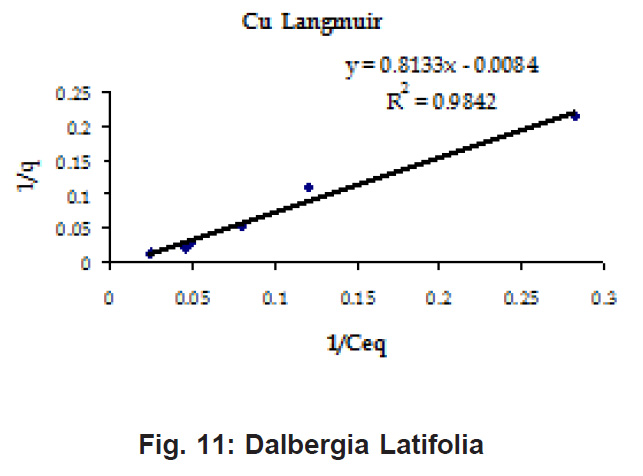
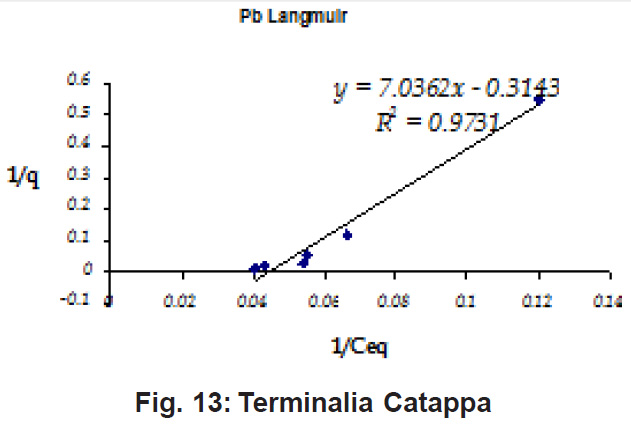
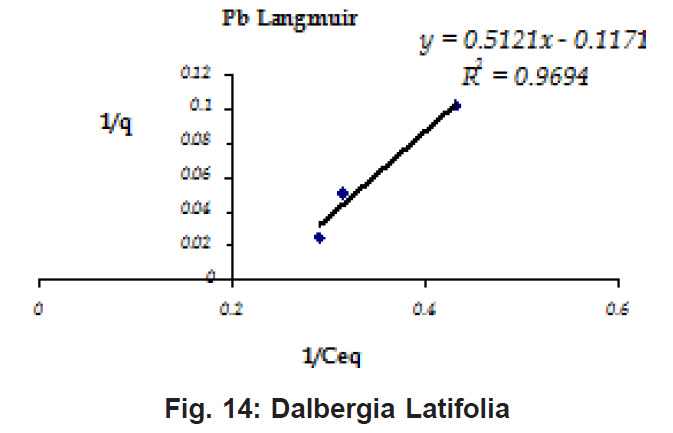
Percent sorption for both the elements was between 95 to 97 % for all the three biomasses except for Pb on Dalbergia latifolia which was 74% (Table 1). Data fitted both Langmuir and Freundlich equation but more so with Langmuir as is evident from r2 values. Also, calculated r2 values match the surface and bonding sites with different energies as is seen in (Salamatinia et al., 2007) where the similar results were observed.
 |
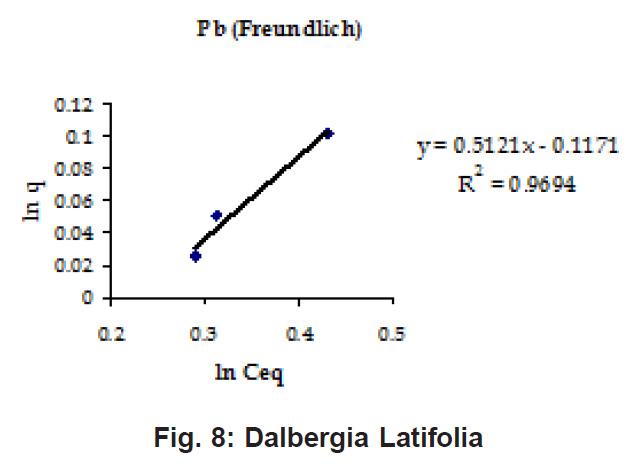 |
Figure 8: Dalbergia Latifolia
Click here to view figure |
 |
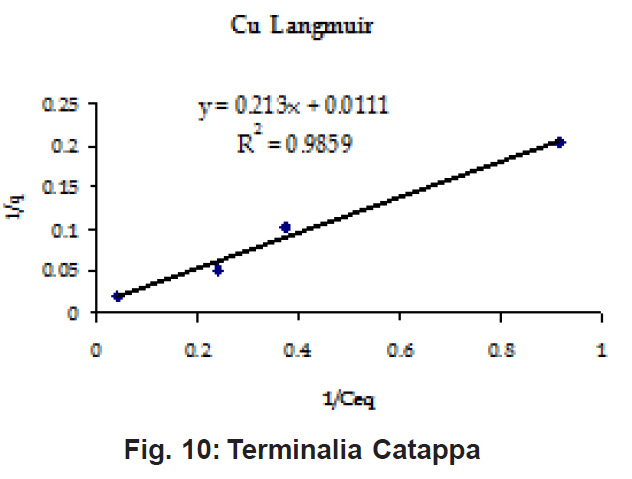 |
Figure 10: Terminalia Catappa
Click here to view figure |
 |
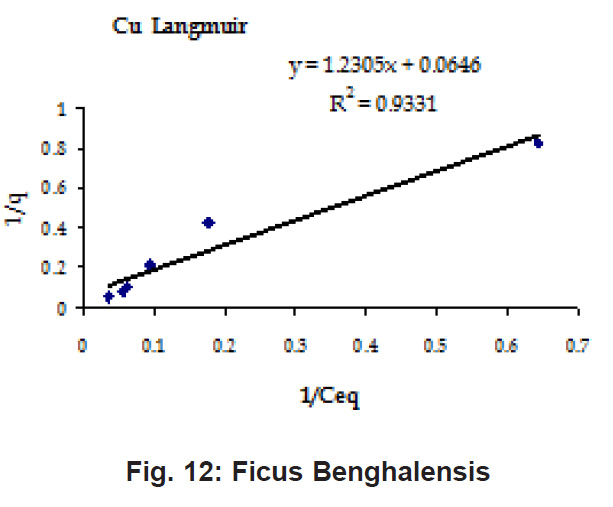 |
Figure 12: Ficus Benghalensis
Click here to view figure |
 |
 |
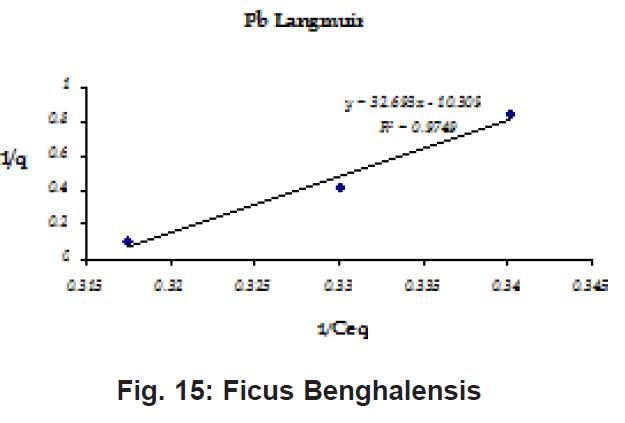 |
Ion-exchange also is playing a significant role as is evident from their leaf composition values determined by XRF (Table 3). Ion-exchange is feasible more in Terminalia Catappa than in Dalbergia latifolia and which in turn can be more than in Ficus benghalensis.
Negative values of Gibbs free energy indicate the spontaneity of the adsorption process between metals and the leaf powders. The standard Gibbs free energy of the biosorption process corresponding to Pb and Cu on the biomass was evaluated using equation described by Babarinde et al. (2008) (Table 4). The negative values of ÄGo indicate the spontaneous sorption by the biomass. As the values are more than -40 kJ/ml (Jnr and Spiff, 2005) chemisorption is implied from the Langmuir graph monolayer coverage can be used to calculate surface area with the following equation:
The dimensionless constant separation factor RL is given by:
RL= 1/ (1+bCo)
It is an essential feature of Langmuir Model where b is the Langmuir constant and Co is the initial metal concentration (mg l-1).For favourable adsorption its values should be between 0 and 1 (Poots et al., 1978). It can be seen that all the values are between 0.02 and 0.7 (Table5) which indicates favourable adsorption.
Conclusion
experimental values (Table 2). This shows that there is monolayer sorption. Cu and Pb show almost similar sorption capacities on the three biomasses. The reason for this preference is that the size of the Cu metal ion which is smaller than Pb ion and thus it can get attached to the powder easily. Sorption was low on Ficus benghalensis but both the cations were equally adsorbed by this biomass without any preference (Fig. 7 to 12). The ‘n’ values should fall in 1-10 range for beneficial adsorption (Kalin et al., 2005). Values are in the range 0.25 to 1.056 suggesting the presence of heterogenous,These three biomasses can be obtained without excessive cost.
Terminalia Catappa leaves show highest adsorption capacity compared to Dalbergia latifolia and Ficus benghalensis. The results show that in very less time, a good amount of the metal can be taken up by the biomass. The present results demonstrate that the Langmuir model fits better than the Freundlich model for the adsorption equilibrium data in the examined concentration range. Langmuir Model suggests monolayer adsorption whereas ÄGo values favour chemisorption. RL values also show favourable adsorption. It is important to note that the biomass works efficiently without any pre treatment.
Terminalia Catappa & Dalbergia latifolia seem to be the promising biosorbents for heavy metals from aqueous solutions.
References
-
S.S. Ahluwalia, D. Goyal. Microbial and plant derived biomass for removal of heavy metals from waste water. Bioresource technology 2006.
-
K.H. Chong, B. Volesky. Description of two-metal biosorption equilibria by Langmuir-type models. Biotechnol. Bioeng. 1995;47:1-10.
-
Elouear, J. Bouzid and N. Boujelben. Removal of nickel and cadmium from aqueous solutions by sewage sludge ash: Study in single and binary systems. Environmental Technology. 2009;30(6):561-570.
-
S.Chaiyasith, P. Chaiyasith, C. Septhu.Removal of Cadmium and Nickel from aqueous solution by adsorption onto treated fly ash from Thailand. Thammasat. Int. J. Sci. Technol. 2006;11:13-20.
-
J. W. Patterson, Industrial wastewater Treatment Technology, second ed. Butterorth Publisher, Stoneham, MA . 1985
-
Z. Aksu., T. Kutsal. A bioseparation process for removing lead (II) ions from waste water by using C. vulgaris. Z Journal of Chemical and Technology Biotechnology. 1991;52(1):109-1181.
-
M. Friedman, A.C. Waiss. Mercury uptake by selected agricultural products and by-products. Environ Sci Technol . 1972;6:457-8.
-
M.A.K.H. Hanafiah, S. Shafiei, MKHarun and M.Z.A. Yahya. Kinetic and thermodynamic study of Cd2+ adsorption onto rubber (Hevea brasiliensis) leaf powder. Mater. Sci. Forum. 2006;517:217-221.
-
M .A. K Hanafiah., W.S. Wan Ngah, H. Zakaria and S.C. Ibrahim, Batch study of liquid-phase adsorption of lead ions using Lalang (Imperata cylindrical) leaf powder. Journal of Biological Sciences. 2007;7(2):222-230.
-
P. Goyal, P. Sharma, S. Srivastava & M. M. Srivastava. Saraca indica leaf powder for decontamination of Pb: removal, recovery, adsorbent characterization and equilibrium modeling. International Journal of Environmental Science and Technology, 5(1): 27-34 (2008).
-
Mahvi, A. H., Gholami, F., & Nazmara, S. Cadmium biosorption from wastewater by Ulmus leaves and their ash. European Journal of Scientific Research, 23(2): 197-203 (2008).
-
A. H.Mahvi, F. Gholami, & S.Nazmara. Cadmium biosorption from wastewater by Ulmus leaves and their ash. European Journal of Scientific Research, 23(2): 197-203 (2008).
-
P. Salehi, B. Asghari and F. Mohammadi. Removal of Heavy Metals from Aqueous Solutions by Cercis siliquastrum L. J. Iran. Chem. Soc. 5: S80-S86 (2008).
-
S. Qaiser, A.R. Saleemi, M.M. Ahmad. Heavy metal uptake by agro based waste Materials. Electronic J. Biotechnol. 10:: 409-416 (2007).
-
P. King, N. Rakesh, S. Beenalahari, Y. Prasanna Kumar, V.S.R.K. Prasad. Removal of lead from aqueous solution using Syzygium cumini L.: Equilibrium and kinetic studies. Journal of Hazardous Materials. 142: 340-347 (2007).
-
P. Hepple. Lead in the environment, Inst. of Petroleum, London (1972).
-
L. Carson, H.V. Ellis, J.L. McCann. Toxicology and monitoring of metals in human, Lewis Publishers Inc., U.S.A. p. 130 (1986).
-
C. Raji and T.S. Anirudhan, Chromium (VI) adsorption by sawdust carbon: kinetics and equilibrium. Indian Journal of Chemical Technology, 4(5): 228-236 (1997).
-
Mohammad Ajmal, Rifaqat Ali Khan Rao, Rais Ahmad, Moonis Ali Khan.Adsorption studies on Parthenium hysterophorous weed: Removal and recovery of Cd(II) from wastewater. Journal of Hazardous Materials. B135: 242-248 (2006).
-
K.G. Bhattacharyya and S.S. Gupta. Pb (II) uptake by kaolinite and monmorillonite in aqueous medium: Influence of acid activation of the clays. Colloid Surf. A: Physicochem. Eng. Asp. 277: 191-200 (2006).
-
Hana Runping, Li Hongkui, Li Yanhu, Zhang Jinghua, Xiao Huijun, Shi Jie. Biosorption of copper and lead ions by waste beer yeast. Journal of Hazardous Materials. B137: 1569-1576 (2006).
-
M. Kalin, W.N. Wheeler, G.Meinrath. The removal of Uranium from mining waste water using algal/microbial biomass. J. Environ.
-
B. Salamatinia, A.H. Kamaruddin and A.Z. Abdullah. Removal of Zn and Cu from wastewater by sorption on Oil Palm TreeDerived Biomasses. J. of Applied Sciences 7(15): 2020-2027 (2007).
-
N.A.A. Babarinde. Adsorption of zinc (II) and cadmium (II) by coconut husk and goat Hair. J. Pure Appl. Sci. 5(1), 81-85 (2002).
-
Michael Horsfall Jnr and Ayebaemi I. Spiff. Effect of metal ion concentration on the biosorption of Pb2+ and Cd2+ by Caladium bicolor (wild cocoyam). African Journal of Biotechnology. 4(2): 191-196, (2005). 26. V.J.P. Poots, G. McKay, J.J. Healy, Removal of basic dye from effluent using wood as an adsorbent. Water Pollut. Control Fed. 50: 926 (1978)
-
26. V.J.P. Poots, G. McKay, J.J. Healy, Removal of basic dye from effluent using wood as an adsorbent. Water Pollut. Control Fed. 50: 926 (1978).






