Thermal stability of copolymers of p-bromophenyl acrylamide-methyl methacrylate
Fahd A.A. Trikistani1 * and I. Zaafarany1
Corresponding author Email: drfahd999@gmail.com
DOI: http://dx.doi.org/10.12944/CWE.5.1.01
Copolymers of p-bromophenyl acrylamide with methyl methacrylate were prepared and characterized using microanalysis and IR spectroscopy. The reactivity ratio values of the copolymerization were calculated using 1HNMR technique. Thermal analyses of the copolymers showed that the stability are intermediate between poly(Pbromo phenyl acrylamide) and poly (methyl methacrylate) homopolymers.
Copy the following to cite this article:
Trikistani F. A. A, Zaafarany I. Thermal stability of copolymers of p-bromophenyl acrylamide-methyl methacrylate. Curr World Environ 2010;5(1):01-08 DOI:http://dx.doi.org/10.12944/CWE.5.1.01
Copy the following to cite this URL:
Trikistani F. A. A, Zaafarany I. Thermal stability of copolymers of p-bromophenyl acrylamide-methyl methacrylate. Curr World Environ 2010;5(1):01-08. Available from: http://www.cwejournal.org/?p=1086
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2010-04-06 |
|---|---|
| Accepted: | 2010-05-12 |
Introduction
It is well known that the thermal stability of polymers has been improved by copolymerization of the primary monomer with traces of a comonomer [AI-Mazroai (2000); Tirkistani (2008) and Tirkistani (2008)]. The poor thermal stability of brominated polymers, due to the weak character of the C-Br bond, has received much attention [EI-Agamey & Diab (1986) and Grassie et al., (1981)]. In this paper, bomopolymers of poly(p-bromophenyl acryiamide) (PBPA) and poly (methyl-methacrylate) (PMMA) and five different compositions of copolymer of p-bromophenyl acrylamide and methyl methacrylate (BP- A-MMA) were prepared, so that the reactivity ratios might be determined using ¹H-NMR method. The thermal stability of the homopolymers and copolymers were examined.
Experimental
Materials
Methyl methacrylate (MMA) (BDH Chemicals Ltd.), stabilized with 0.1 % by hydroquinone was washed with a small amount of sodium hydroxide solution, separated with a separating funnel, distilled in vacuum line and dried over anhydrous sodium sulphate and stored below ~18°C.
Acryloyl chloride (AC) (Aldrich Chemical Co., Inc.) was used with out further purification. It was stored below -18°C in a tightly glass-stoppered flask.
2,2-Azobis isobutyronitrile (AIBN) (Aldrich Chemical Co., Inc.) was used as initiator for polymerization. It was purified by dissolving in hot ethanol and filtering [Diab (1983)]. The solution was left to cool, the pure material then being collected by filtration and dried under vacuum.
p-Bromoaniline (BA) (BDH Chemicals Ltd.), was purified by distillation under atmospheric pressure [Wei & Hsueh (1968)].
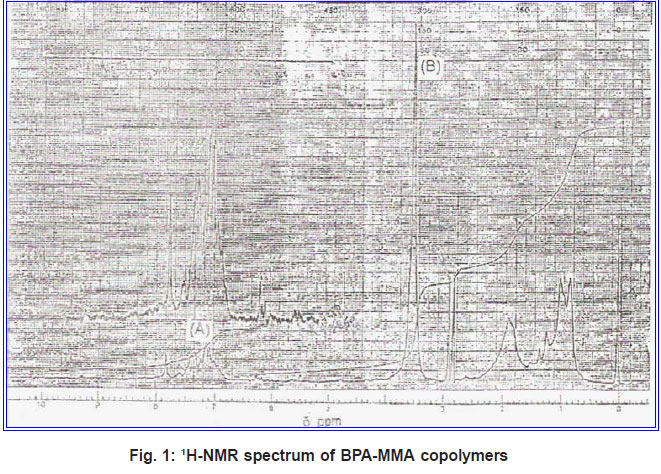 |
Figure 1 |
Preparation of Monomers and Polymers
p-Bromophenyl acrylamide (BPA) monomer was prepared by the reaction of equimolar amounts of AC and BA using dry benzene as solvent in an ice bath. This process is similar to the one reported for the preparation of acryloyl hydrazine, N-(B-ethyl-amino )acrylamide and N-2( 6-aminopyridine )acrylamide [Takrony (2000)
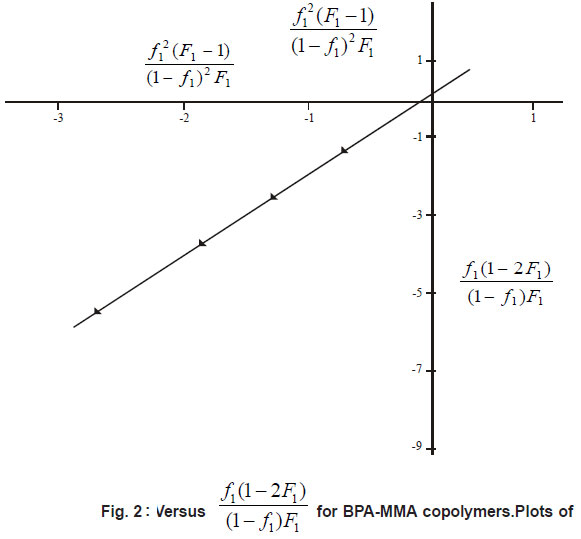 |
Figure 2 click here to view figure |
Poly(p-bromophenyl acrylamide) (PBPA) and poly(methyl methacrylate) (PMMA) homopolymers were prepared by refluxing BA or MMA monomers with dimethylformamide (DMF) (50/50 v/v) as solvent and 0.2 w/v % AIBN as initiator for two hours. The polymer was precipitated by pouring it in distilled water and dried in a vacuum oven for several days at 40°C.
Copolymers of BPA-MMA were prepared using 0.2 w/v % AIBN as free radical initiator and 5O/50 (v/v) DMF as solvent. Five different copolymer compositions of BP A-MMA were prepared, so that the reactivity ratios might be determined. Polymerization was carried out to about 10% conversion. The polymers were precipitated by pouring into a large excess of distilled water, filtered and dried in a vacuum oven at 40°C for several days.
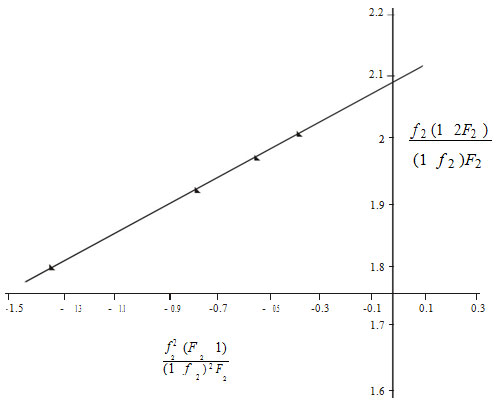 |
Figure 3 click here to view figure |
Analytical Techniques Infrared Spectroscopy (IR)
Infra-red spectra were recorded on Pye Unicam SP 2000 spectrophotometer, for the homopolymers and copolymers
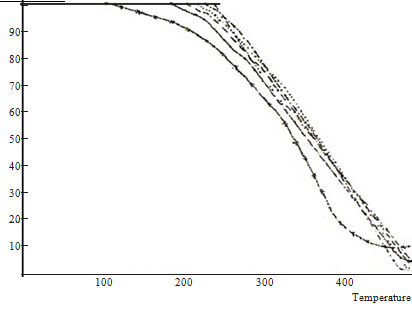 |
Figure 4 click here to view figure |
Nuclear Magnetic Resonance Spectroscopy (NMR
1H-NMR spectra were obtained using a Varian EM 390 90 MHZ spectrophotometer with integration and 20 mg samples. The polymers were dissolved in 1 mL of CDCl3. The integral obtained for each sample was used for determination of the polymer compositions
Microanalysis
Nitrogen contents were determinated by the Microanalytical Unit at King Abdel Aziz University, Saudi Arabia.
Thermal Method of Analysis Tbermogravimetry (TG)
T.G.A. measurements were carried out with a Mettler TO 3000 apparatus.
Finely powdered (- 10 mg) samples were heated at 10°C /min in a dynamic nitrogen atmosphere (30 mL/min.); the sample holder was boot-shaped, 10 mm × 5 mm x 2.5 mm deep and the temperature measuring thermocouple was placed 1 mm from the sample holder.
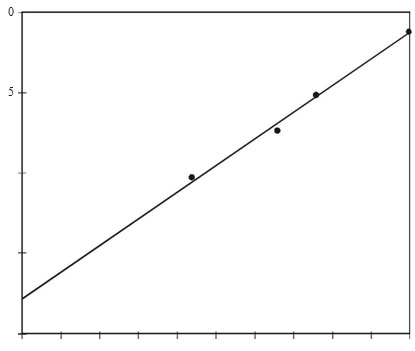 |
Figure 5 click here to view figure |
T.G.A was also used for the determination of rates of degradation of the homopolymers and copolymers in the initial stages of decomposition. The activation energies were obtained by the application of Arrhenius equation.
Results and Discussion
Characterization of PBP A Homopolymers and BP A-MMA Copoliymer
BP A monomer was prepared by the reaction of equimolar amounts of AC and p-bromophenol in dry benzene until the evolution of hydrogen chloride ceased forming a brown powder of BPA monomer (M.W. 226). (Microanalysis, found N, 6.2% calcd. for C9H8ONBr, N 6.19%).
PBPA homopolymers was prepared by free radical initiation of BPA using 0.2 w/v % AIBN as initiator to (~ 10 %) conversion. The IR spectrum of PBPA homopolymers shows two medium broad bands at 3290 and 3441 cm-l assigned to symmetric and asymmetric stretching vibrations of the amino group. The band at 1680 cm-l is assigned to the antisymmetric stretching vibration of amidic carbonyl group. The bands at 1600, 1545 and 1440 cm-l are assigned to the v (C-H), v(C=C) and v(C-C) bonds, respectively [EI-Sonbati et al., (1991)]. The C-H in plane deformation in the region 1225-1045 cm-1, the ring breathing at 995 and 1005 cm-l the out-of-plane C-H deformation vibration between 775 and 750 cm-1 and the C-C out-of-plane deformation at 500 cm-1 are assigned.
The IR spectrum of BPA-MMA copolymer exhibits a characteristic strong band at 1730 cm-l assigned to the antisymmetric stretching vibration of carbonyl group of MMA. There is no change in the position of the bonds of amino group of BPA units in the copolymers.
Determination of Reactivity Ratios of BPA-MMA Copolymers
Five different compositions of the BPA-
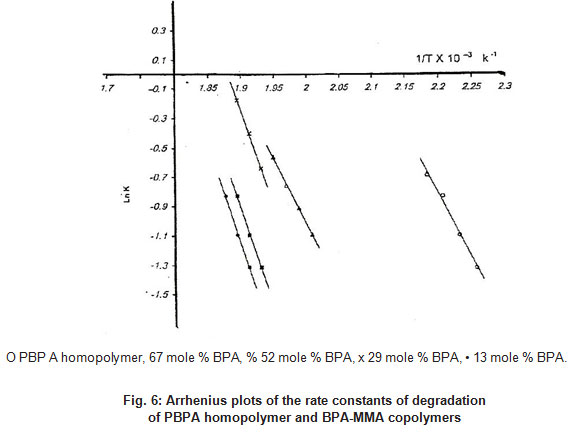 |
Figure 6 click here to view figure |
MMA copolymers with 67, 52, 43, 29 and 13 mole
BPA units prepared by free radical initiation using 0.2 w/v % AIBN and DMF (50/50 v/v) as solvent. The polymerization were carried out at 60°C to about 10% conversion.
The integration of 1H-NMR spectrum from each sample of the copolymer was used for the calculation of copolymer composition. The monomer composition of the copolymer can be calculated from the ratio of these integrals which are proportional to the number of protons that contribute to the peaks. This method was already used for the determination of reactivity ratios for different copolymers [Grassie et al., (1986) and Grassie et al., (1965)]. Figure 1 shows the 1H-NMR spectrum of BPA-MMA copolymers. There are three characteristic peaks at δ 0.85-1.05, 1.88-1.94 and 2.77.2.86 ppm for CH3, CH2 and CH protons, respectively [EI-Sonbati (1990)]. The amino proton at δ 7.85 ppm was disappeared on addition of D2O. Peak A at δ 7.15 and 7.38 ppm which is a composite peak, due to two protons in the ortho-position and protons in meta-position of the benzene ring of BP A in the copolymer, respectively [Mochel (1967)]. Peak B at δ 3.65 ppm is due to –OCH3 protons of the MMA unit. Dividing the integral values due to peak A by four and peak B by three, the monomer composition of the copolymer -can be calculated. By knowing the number of moles of the monomer mixture and the molar ratio of the copolymer, reactivity ratios can be calculated by means of the following equation [Billmeyer (1971)].

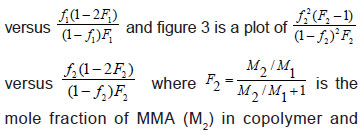
mole fraction of MMA (M2) in copolymer and is the mole fraction of M2 in feed. From the slopes and intercepts in Figure 2 and 3 reactivity ratio values for BPA-MMA copolymer are r1(BPA) = 0.27±0.01 and r2(MMA) = 1.8±0.2.
Thermal methods of analysis Thermogravimetry (TG)
TGA curves of PBP A and PMMA homopolymers and copolymers BP A-MMA are shown in Figure 4. There are two degradation stages of PBPA homopolymer, the first starts at ~ 110°C with a weight loss of ~ 52%. The second stage starts at ~ 310°C with a weight loss of ~ 40%. PMMA homopolymers degrades in two stages. The first
Table 2: Activation energies of the thermal degradation of PBPA and PMMA homopolymers and BPA-MMA copolymers
|
Polymer |
Activation energy |
|
BPA mole% |
(Ea)kJ/mol |
|
PBPA |
69.1 |
|
67 |
72.7 |
|
52 |
78.2 |
|
43 |
84.8 |
|
29 |
89.3 |
|
13 |
96.1 |
|
PMMA |
116.4 |
step starts at ~ 260°C with a weight loss percentage of ~ 38%. The second degradation stage starts at 350°C with a weight loss percentage of 56%. There are three TGA degradation stages for all the BPA-MMA copolymers. The volatilization temperature starts at ~ 160, 182, 198, 215 and 256°C for the copolymers 67, 52,43,29 and 13 mole % BPA units, respectively.
Table 1 lists the percentage weight losses and the maximum rate of weight loss shown in the derivative thermogravimetry DTG. The TGA curves show that the stabilities of the copolymers are intermediate between those of the homopolymers. To illustrate the initial stages of breakdown more clearly, a comparison study of weight loss percentage at definite temperature with copolymer composition give a clear picture on the relative stability of the entire composition range. Smooth changes in stability with composition is well demonstrated in Figure 5 is plotted against composition. The most clearly defined feature of the reaction is the increase of stability towards PMMA homopolymer.
The effective activation energies of thethermal degradation of PBP A and PMMA homopolymers and BP A-MMA copolymers were determined from the temperature dependence of the chain rupture rate. The rate constant of the thermal degradation was plotted according to Arrhenius relationship (Figure 6). Table 2 lists the activation energies of PBP A and PMMA homopolymers and BP A-MMA copolymers. The activation energy of PBPA homopolymers is smaller than of the copolymers and PMMA homopolymers. Therefore PBPA homopolymers -will undergo decomposition more readily than BP A-MMA copolymers and PMMA homopolymers.
References
2. Billmeyer, F. W. Jr., Textbook of Polymer Science, New York, Wiley Interscience, (1971) Chap 11, 328 - 354.
3. Diab, M.A., Thermal Stability of Poly(vinyl bromide) and Vinyl bromide-methyl acrylate copolymer, J. Polym. Sci., Polym. Chem. Ed. (1983) 21: 3249 - 3253.
4. EI-Agamey, A. A. and Diab, M. A., Thermal Stability of Poly(B-bromostyrene) and copolymer of B- bromostyrene with methyl methacrylate, J. Thermal Anal., (1986) 31: 239-245.
5. EI-Sonbati, A. Z., Synthesi and properties of 7-formyl-8-hydroxyquinoline and its transition metal complexes. Transition Met. Chem., (1990) 15:222-229.
6. EI-Sonbati, A. Z., Kotb, M. A. and Killa, H.A., Structural chemistry of Poly(2-acrylamido-1,2 diaminobenzene) complexes. Bull. Soc.Chim. Fr., (1991) 128: 623-636.
7. Takrony, K. M., Physico-chemical studies on some polymer complexes, M.Sc. Thesis,Umm AI Qura University (2000).
8. Grassie, N., Torrance, B. J. D.; Fortune, J. D.and Gemmel, J. D., Stability and degradation of methyl methacrylate-methyl acrylate copolymers, Polymer, (1965) 6: 653-659.
9. Grassie, N.; Jhonstone, A. and Scotony, A.,Thermal degradation of bromine containing polymers, Eur. Polym. J., (1981) 17: 589-599.
10.Grassie, N.; Diab, M. A. and Scotony, A.,Thermal degradation of bromine containing polymers, Polym. Deg. And Stab., (1986) 16: 361-369.
11. Mochel, V., Presented at a meeting of the division of rubber chemistry, Amer. Chem.Soc., Montreal, Canada (1967).
12.Tirkistani, F. A. A., Thermal stability of Poly(N-[3-(5-amine-1,2,4-triazolo ]acrylamide homopolymer and copolymer of N-[3-(5-amino-l,2,4-triazolo ]acrylamide withmethyl methacrylate, Mans. J.Chem., (2008) 35:177-193.
13. Tirkistani, F. A. A., Thermal stability of Poly(N-[3-(5-amino--1,2,4-triazolo]acrylamido and copolymer of N-[3-(5-amino-l,2,4-triazolo)]acrylamido with methyl acrylate,Mans. J. Chem., (2008) 35: 195--208.






