Physicochemical studies of ground water at different areas in Jalgaon city (M.S.) India
M.S. Mustaqeem1 * and G.A. Usmani2
DOI: http://dx.doi.org/10.12944/CWE.5.1.20
Present work deals with the study of physicochemical parameters of ground water samples in Jalgaon city at different sampling stations during 2008 – 2009 in pre monsoon, monsoon and post monsoon seasons. The observed values of various physicochemical parameters of water samples were compared with standard values recommended by W.H.O. It was found that the concentration of total alkalinity, calcium, magnesium, pH, zinc, total hardness and total dissolved solids are within permissible limits of W.H.O. But the concentration of cadmium and chromium in the present water samples exceeds the maximum permissible limit of W.H.O. However, the sample collected at station no.2, bore well water of Salar Nagar, shows the value of Hardness and TDS more than maximum permissible limits of W.H.O. Therefore this water can be used for household purpose, but is harmful for drinking.
Copy the following to cite this article:
Mustaqeem M. S, Usmani G. A. Physicochemical studies of ground water at different areas in Jalgaon city (M.S.) India. Curr World Environ 2010;5(1):127-129 DOI:http://dx.doi.org/10.12944/CWE.5.1.20
Copy the following to cite this URL:
Mustaqeem M. S, Usmani G. A. Physicochemical studies of ground water at different areas in Jalgaon city (M.S.) India. Curr World Environ 2010;5(1):127-129. Available from: http://www.cwejournal.org/?p=1124
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2010-03-02 |
|---|---|
| Accepted: | 2010-04-23 |
Introduction
Water is the most vital source for all kinds of life on the earth.1 In India about 96 % of water resources is used for agriculture, 3% for domestic use and 1 % for industrial activity.2 In India ponds, rivers and ground waters are used for domestic and agriculture purposes. The quality of water may be described according to their physicochemical and microbiological characteristics.3 However contamination of ground water used for drinking purpose can affect public health.4 so the physico-chemical study of ground water is necessary. Trace metal ions have important roles in life, involving a wide spectrum of activities. Thus the determinations of heavy metal ions becomes increasingly important.5
Experimental
Study Area
Jalgaon city lies between 20 0 to 210 North Latitude land 74 0 55’ & 76 0 28’ east longitude land on the northern border of the state of Maharashtra.6 The study was undertaken at three different areas of Jalgaon city considering the surroundings and distance. The sample water was collected from bore well. The sampling stations selected for the present study are Sample station
1 Bore well water of Iqra campus, Shirsoli road, Jalgaon. Sample station
2 Bore well water of Salar Nagar, Jalgaon. Sample station
3 Bore well water of Apna Ghar colony, Jalgaon.
The analysed data were compared with standard values recommended by W. H. O.
Water Samples and Chemicals
The water samples were collected in clean polythene bottles which were thoroughly rinsed with samples and tightly sealed and labelled after collection. The method applied by R. Shyamala et. al.7 has been used. All the chemicals used were of A R grade.
Methodology
The water samples were collected from sampling stations mentioned above in pre monsoon, monsoon and post monsoon seasons in the year 2008-09. Temperature, pH ,Total Dissolved Solid and Electrical Conductance were measured by using calibrated thermometer 1/10, Equiptronics digital pH meter model EQ-610, digital TDS meter model 514 and digital conductivity meter model EQ 664 respectively. Alkalinity, Hardness, Ca and Mg were measured volumetrically by standard procedures. Heavy metals such as chromium, cadmium, zinc and iron were determined by Atomic Absorption Spectrophotometer (Unicom, U.K. model -52). The method for determination of Fe in natural and mineral waters by Flame Emission Atomic Absorption Spectrophotometer was used, as suggested by Stasys and Laura.8
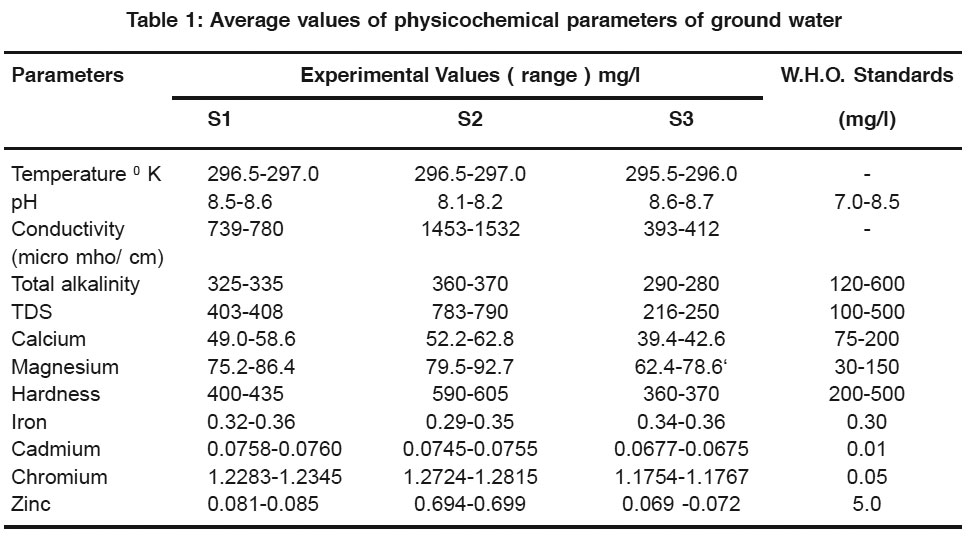 |
Table 1: Average values of physicochemical parameters of ground water Click here to view table |
Results and Discussion
The results of physicochemical analysis of the ground water samples S1 to S3, collected from 3 different places of Jalgaon city are compared with standard values and presented in Table 1. The observed pH value ranges from 8.1 to 8.7 showing that the present water samples are slightly alkaline. These values are within maximum permissible limit prescribed by W. H. O. 9 Generally pH of water is influenced by geology of catchment area and buffering capacity of the water. The electrical conductance was ranging from 393 to 1532 micro mho/ cm. The observed TDS value of sample 1 & 3 is from 216-408 mg/l. This is under the permissible limit of W. H. O. It was observed that sample no. 3 crosses the maximum permissible limit having TDS more than 500 mg/l. High TDS in ground water may be due to ground water pollution when waste waters from both residential and dyeing units are discharged into pits, ponds and lagoons enabling the waste to migrate down to the water table.10 Similarly hardness of sample no. 1 & 3 is also in the prescribed limit of W. H. O from 360-435 mg/l. However, the value of hardness of sample no. 2 was 590-605 mg/l., which is well above the maximum permissible limit prescribed by W. H .O (500 mg/l). The maximum permissible level of total alkalinity is 600 mg/l. The value of total alkalinity of all the water samples ranges from 280-335mg/l. This is under the prescribed limit of W. H. O. The value of total alkalinity in water provides an idea of natural salts present in water. The cause of alkalinity is the mineral which dissolves in water from soil. The various ionic species that contribute to alkalinity include bicarbonate, hydroxide, phosphate, borate and organic acids. Calcium concentration was found to vary from 39-62 mg/l. The higher limit of calcium concentration for drinking water is specified as 75 mg/l. The calcium hardness observed in all the 3 stations is within desirable limit. The concentration of magnesium was found to vary from 62-92 mg/l. The limit of magnesium concentration for drinking water is specified as 30-150 mg/l. Thus the magnesium concentrations of water samples are within the prescribed level of W.H.O.
The study of heavy metals, iron and zinc in all the water samples were recorded from 0.29-0.36 mg/l and 0.069 to 0.699 mg/l respectively. These values show that the concentration of iron and zinc are within permissible limit (0.30 mg/l. and 5.0 mg/l respectively). Cadmium ranges from 0.0677 to 0.0760 mg/l were exceeds the prescribed limit of W. H. O. which is 0.01 mg/l for cadmium. Similar values of cadmium, (more than 0.01 mg/l) was recorded by S. R. Gaikwad11. While the concentration of chromium ranges from 1.1754 to 1.2835 exceeding the prescribed limit of W. H. O. for chromium which is 0.01 mg/l.
Conclusion
The physicochemical study of water samples from three different stations of Jalgaon city shows that all physicochemical variables are within the highest desirable limit or maximum permissible limit set by W.H.O. except chromium and cadmium. The results of the present study suggest that the ground water of areas under study need treatments to minimize contaminations for domestic and drinking purpose. However, samples at station no.2 also show high values of TDS and hardness compared to prescribed value of W.H.O. Therefore in the light of P. Yadaiah’s classification of ground water, it can be concluded that the ground water sample of station no.2 is not suitable for drinking purpose but can be used for other household purpose.12
References
- Trivedy R. K. And Goel P.K., Chemical and Biological methods for Water Pollution studies, Environmental Publications, India, (1986) 36-72.
- Ashok C., Fundamentals of Environmental science, Anmol prakashan, New Delhi, India, 45 (1990).
- Bhandari N. S. and Kapil N., E-journal of chemistry, (2008) 5(2):342-346
- Allen H. S., Elenao O. L., and Mahfuzar R., Bulletin of World Health Organisation, (2000) 78(9): 1093-1102.
- Erdal K. and. Rehber A., The Japan Society of Analytical Chemistry, (2002) 18: 917-921.
- Gupta G. R. and Chaudhari G. R., Asian Journal of Chemical and Environmental Research, (2008) 1(1):59-62.
- R. Shyamala, M. Shanthi and P. Lalitha , E-Journal of Chemistry, (2008) 5(4): 924-929.
- Sasys T., Laura A., Steponneneiene S., and Rolandas K., Journal Serb. Chem. Soc., (2004) 69(5):393-402.
- World Health Organisation, Guidelines for drinking water quality-I , Recommendations, 2 nd Edition, Geneva WHO, 19 (1993).
- Rani D.F.G., Geetha S. and Ebanazar J., J. Pollut Res., (2003) 22(1): 111-115.
- Gaikwad S. R., and Thorat S. R., Bulletin of Environmental Science, (2006) 4(1): 71-75.
- Subrahmanyam K., and Yadaiah P., Hydrology Journal, (2001) 9: 297-312.






