Evaluation of adsorption efficiency of Ferronia elephantum fruit shell for congo red retrieveal from aqueous solution
U.E. Chaudhari1 *
DOI: http://dx.doi.org/10.12944/CWE.5.1.38
Congo Red adsorption from an aqueous solution on Ferronia elefuntum fruit shell (FEFS) has been studied experimentally using the batch adsorption method. The operating variables are pH, initial dye concentration contact time. Adsorption isotherm (Langmuir and Freundlich) and kinetics model were studied. The adsorption capacity of FEFS was found to increasing with increase in temperature. Thermodynamics parameters such as “G, “H, and “S for adsorption were evaluated. Adsorption of Congo Red on FEFS found to be endothermic process. The aim of present work is to study the effectiveness of the adsorbent dyes from their aqueous solution and the removal of color from textile and various industrial waste water.
Copy the following to cite this article:
Chaudhari U.E. Evaluation of adsorption efficiency of Ferronia elephantum fruit shell for congo red retrieveal from aqueous solution. Curr World Environ 2010;5 (1):213-216 DOI:http://dx.doi.org/10.12944/CWE.5.1.38
Copy the following to cite this URL:
Chaudhari U.E. Evaluation of adsorption efficiency of Ferronia elephantum fruit shell for congo red retrieveal from aqueous solution. Curr World Environ 2010;5 (1):213-216. Available from: http://www.cwejournal.org/?p=1160
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2010-04-12 |
|---|---|
| Accepted: | 2010-05-29 |
Introduction
The retrieval of color from industrial effluent is a major problem as restriction become more stringent. Effluents from textile pulp and paper industries are highly colored due to residual dyes, and thus lower the aesthetic value structure and are toxic and harmful for aquatic and human life. The presence of color in water inhibits the growth of aquatic fauna and flora by reducing light penetration. Various techniques such as chemical Coagulation Bio – sorption, Oxidation using ozone and adsorption have been generally employed for ret rival of color, Adsorption is one of the most effective physical process and has a great potential for the removal of dyes from wastewater. The aim of this study was to prepare activated carbon from Ferronia elephantum Fruit Shell and adsorption isotherm was developed for Congo red cycle. Which can be readily used to designing purpose in pollution amendment and control.
Material and Methods
Adsorbent Preparation
The adsorbent Ferronia elephantum Fruit shell (FEFS) was collected from the Pandhari forest situated in between warud and Padhurna. The Ferronia elephantum Fruit Shell was first dried at a temperature of 160°C for six hours. After grinding it was then soaked overnight in 0.1 N NaOH solution to remove the lignin content, excess alkalinity was then neutralized with 0.1 N HCI solution. It was washed with distilled water several time till the wash water become colorless. Then it was kept in muffle furnace at 130°C for 6 hrs. it was sieved to obtain average particle size of 200 mesh. Finally it was dried again in an over at 50°C for hours. The adsorbent was then stored in desiccators for final studies.
Adsorbate Preparation and Batch Study
Stock solution (1000mg/L) of Congo Red was prepared by dissolving 1 gm of dye in 1000 ml of double distilled water. The stock solution were diluted with double distilled water to obtain required standard solution. The dried amount of 0.2gm. of FEF. Shell was take in 250 ml reagent bottle and standard solution (100ml) containing various concentration of Congo Red dye was added and system is equilibrated by shaking the contents of the flask at room temperature. The adsorbent and adsorbent were separated by filtration and filtrate was determined by spectrophotometer at 495 nm a against a reagent blank. The spectrophotometer systrornic(model 104) was used to measure the concentration of Congo Red.
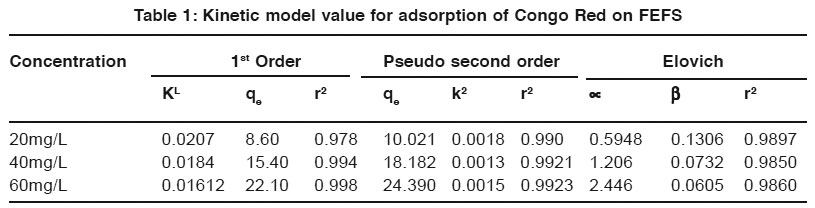 |
Table 1: Kinetic model value for adsorption of Congo Red on FEFS Click here to view table |
Effect of pH on the scavenging the dye was studied using 100ml dye solution having 40 mg/L initial concentration. Effect of initial concentration. Agitating time and adsorbent dose was also studied.
Result and Discussion
Effect of Initial Dye Concentration & Contact Time
The initial concentration of Congo Red solution was varied from 20,30,40,60 mg/L and batch experiments were carried out by taking 200 ml of this solution with dried 200mg on the adsorbent and the system is equilibrated by shaking the contents of the flask at room temperature equilibrium reached in 2 hours. Final concentration of Congo Red was determined by spectrophotometer at 495 n.m. the percentage removal of Congo Red was observed to be 87%. To establish equilibrium time for maximum uptake and to know the kinetics of adsorption process. The adsorption of Congo Red on adsorbent was studied as a function of contact time. Percentage removal of dye is found to decrease with increase in dye concentration. From contact time data it may be seen that dye removal is very rapid during initial period of contact and the maximum are reached within the first 30 minutes removal.
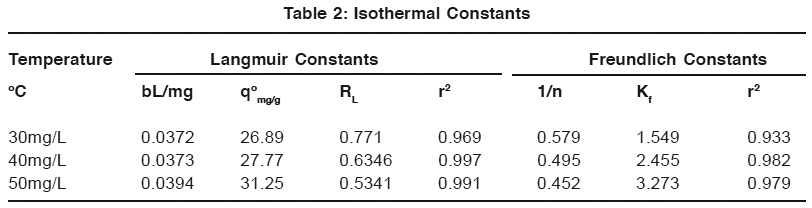 |
Table 2: Isothermal Constants Click here to view table |
Effect on pH
The adsorption capacity of Congo Red as a function of pH. It was observed that percentage removal of Congo Red is maximum of pH = 8 and then decrease with increase of pH.
Effect on Sorbent Dosage
Batch sorption studies were performed to determine the effect of sorbent dosage on Congo red removal. The percent removal increase rapidly and reaches about 95% For 100% removal of the Congo Red the dosage required is 300mg/50ml for the initial concentration of 50mg/L at pH = 8.0
Sorption Kinetics
The rate of adsorption of Congo Red on Ferronia elephantum Fruit Shell was studied by using the first order kinetic model, Pseudo second order kinetic and Elovich models are used to test the experiment data.
First Order Kinetics
The rate of adsorption of Congo Red on Ferronia elephantum Fruit Shell was studied by using the first order rate equation proposed by Lagergren. It is found that as initial dye concentration increase Lagergren rate constant decrease, This indicates that, adsorption does not follow the 1st order kinetics.
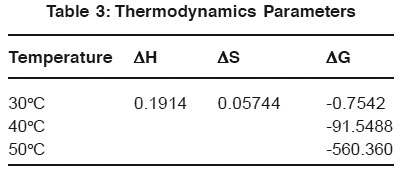 |
Table 3: Thermodynamics Parameters Click here to view table |
Pseudo Second Order Models
Pseudo Second order models showed that, Rate constant K2 is almost constant at different initial concentration which is shown in Table 1. this indicate that adsorption of Congo Red. On Ferronia elephantum Fruit Shell obey the 2nd order kinetics. Also the concentration of Congo Red increasing from 20mg/L to 60mg/L equilibrium sorption capacity qe increase.
Elovich Model
Adsorption of Congo Red an Ferronia elephantum Fruit Shell are shown. A linear relationship is obtained betn the amount of Congo Red, Adsorbed qt and Int. from the table 1, show that value ∝ and β varied as a function fo Congo Red concentration. As the concentration of Congo Red increase from 20mg/L. to 6mg/L. value of ∝ increase and β decrease. This favored the adsorption phenomenon.
Isotherm Modeling
Langmuir Adsorption Isotherm
The Langmuir sorption isotherm is shown in Table 2. Qo values found be comparable with commercial activated carbon. Value of RL lies between 0 and 1 indicate the favorable adsorption. It indicates the applicability of Langmuir adsorption isotherm. The calculated value r2 confirm the applicability of Langmuir adsorption isotherm.
Freundlich Adsorption isotherm
Freundlich plot for the adsorption of Congo Red on Ferronia elephantum Fruit Shell is given in table 2. it was that the values of adsorption intensity 1/n <1, reveal the applicability of freundlich adsorption.
Thermodynamics Parameters
The influence of temperature upon the adsorption rate was investigated at 30°C 40°C and 50°C it is observed that mass of the Congo Red. Adsorbed per unit mass of adsorbent increase with increasing temperature. The negative value of free energy change G indicates the feasibility and spontaneous nature of adsorption of Congo Red.
H value suggests endothermic nature of Congo Red on Ferronia elephantum Fruit Shell. Positive value is Sis due to increase randomness during adsorption of Congo Red.
Conclusion
- Ferronia elephantum Fruit Shell was studied as good adsorbent of removal of Congo Red. The removal is found rapid in initial stage followed by slow adsorption up to saturation level. It also depend an initial concentration of adsorbate and agitating time.
- The present work on adsorption process is in good agreement with Langmuir & Freundlich isotherm indicating monolayer monolayer adsorption process.
- The result of adsorption process reveals that at pH = 8.0, of Congo Red uptake capacity is better.
- The adsorption of Congo Red an Ferronia elephantum Fruit Shell followed the Pseudo second order model and Elovich model.
- Study of temperature effects on Freundlich parameter reveals increasing trend in adsorption capacity with increase in temperature. It followed the endothermic process.
- it is calculated that the adsorbent prepared from Ferronia elephantum Fruit Shell could be exploited for commercial applications. Regeneration studies are not necessary with the view that the cost of the adsorbent is very low and it can be disposed of safely.
References
- B.D.Gharde, S.B. Gholse, P.V.Patil “Revoval of Cu(II) and Ni(II) from solution using Ferronia elephantum Fruit Shell” proceeding of NSAM, (2004) 116-119.
- V.W.Lhangan, D.B.Bakar and S.S.Dara effectiveness of terminalia bellerica bark for scavenging zincions chem.. Environment Res (1992) 1(1): 87-94.
- P.R. Rampure and P.V.Patil, “use of palsa Bark substrate for the recovery of Cu, Pb, Zn,Ni from waste water” Jr.of industrial pollution control, (1996) 12(1).
- R.bankar and M.Arivazhnan, “Secondary baggase pith (SBP) as adsorbents for color removal from textile wastewater” RAWM (2001).
- C. mary Sukanya and A.V.S. Prabhakara Rao, “Color removal from waste effluents using waste activatged carbon” R.A.W.M. (2001).
- Rashmi Sanghi and Ajay Singh, “ Comparative Decolorisation study of Malachite Green Dye solution using synthetic and natural Cwagulaton” Res. J. Chem. Environ.5(2).
- Rashmi Sanghi and bani Bhattachrya, “Decolourisation of malachite Green Dye solution using trees barks as low cost adsorbents” Journal IAEM (2002) 29: 129-135.
- Daga Kailash, Gehlot Poonam and Mehta Rishika, “Comparative study and Treatment of synthetic Dye water using poly vinyl. Alcohol coated activated wood charcoal as adsorbent” Res. J.Chem. Environ. (2007) 11(4).
- Geetha, A. Sivakumar P., “Adsorption of Acid blue from an aqueous solution on to activated areca nut shell carbon : Equilibrium, kinetic and thermodynamics studies” Res. J.Chem. Envirm.(2009) 13(1).
- Lagergren, S and Bil K, Svenska Vatenskapsakad hand,(1998) 24.
- Chien S.H.and Clayton W.R. Application of Elovich equation to kinetic of phosphate release and sorption of soils, soil sci. Am. J., (1980) 44: 265-268.






