Removal of iron from water using modified coconut shell charcoal as adsorbent
K. S. Beenakumari1 *
1
Quality Control Sub Division Lab, Kerala Water Authority,
Thiruvananthapuram,
India
Copy the following to cite this article:
Beenakumari K.S. Removal of iron from water using modified coconut shell charcoal as adsorbent. Curr World Environ 2009;4(2).
Copy the following to cite this URL:
Beenakumari K.S. Removal of iron from water using modified coconut shell charcoal as adsorbent. Curr World Environ 2009;4(2). Available from: http://www.cwejournal.org/?p=971
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2009-04-02 |
|---|---|
| Accepted: | 2009-06-25 |
Introduction
Iron is one of the major impurities that is commonly found in many sources of water. Iron deposited in the distribution system may promote the growth of microorganisms leading to high contamination in drinking water.1 Iron pipes may release corroded iron in water. Chlorine and Bleaching powder added to drinking water as a germicide, oxidizes and corrodes iron. The iron content is prescribed to 0.3 ppm or less as a drinking water quality standard.2
Although iron is an essential mineral helping in the transportation of oxygen in the blood, its presence in ground water above a certain level makes water unusable due to its metallic taste discoloration, odor, turbidity and staining of laundry3. Iron "overload" in drinking water may cause vomiting, bleeding and circulatory disorders.4 Whenthe iron combines with tea, coffee and other beverages, it produces an inky, black appearance and a harsh, unacceptable taste. Vegetables cooked in water containing excessive iron turn dark and look unappealing.5
Iron is mainly present in water in two forms either in soluble ferrous iron or insoluble ferric iron. Water containing ferrous iron is clear and colorless, because the iron is completely dissolved. When exposed to air, the water turns cloudy and a reddish brown substance begins to form. This sediment is the oxidized or ferric form of iron that will not dissolve in water.5 The rates of oxidation are not rapid and this reduced form can persist for sometime in aerated water. This happens when the pH is below 6. In addition, iron can form stable complexes with humic and tannic substances in water that can be even more resistant to oxidation than the inorganic species alone.6
Determination of iron is very important in explorations of new water supplies, particularly from bore well and other surface water sources. The drinking water can be rejected only on the basis of excess iron present in it. When water-containing iron above 0.3 ppm, then it is necessary to decide whether chemical treatment can remove the excess iron and if it seemed to be feasible, the option for best chemical treatment is to be done.
Aeration is the most common method for removal of iron from ground water.3 During the process of aeration, the soluble ferric iron is converted to insoluble ferric state, which can easily be separated from the system. The iron content in surface water is very low due to natural aerial oxidation of iron into its higher oxides, which subsequently separate out. There are various methods for removing iron from ground water. They are ion-exchange method,7 oxidation with oxidizing agents such as chlorine and potassium permanganate, 8, 9, supercritical fluid extraction,10 bioremediation, 4, 11 treatment with lime stone.12,13 magnetic separation.9 and use of activated carbon and other filtering materials.14,15
The present study aims to develop a cheap and easy technology to prevent health disorders due to the presence of excess iron in drinking water. The iron removal system is developed by selecting coconut-shell charcoal as the substrate material. MnO2 is effectively incorporated on the coconut shell charcoal and it is used for adsorbing iron from contaminated water. The developed technique seemed to be very effective in reducing the iron concentration below 0.3 ppm.
Experimental
Substrate material
Coconut shell charcoal is prepared by heating half splitted coconut shell at a temperature of 900 °C for 4 hours using a muffle furnace. The prepared coconut shell charcoal is pulverized using a ball mill and sieved using standard sieves to obtain the required particles size. The proximate analysis of the coconut shell charcoal is as follows:
Table 1: Proximate composition
|
S. No. Parameter Composition (%) |
S. No. Parameter Composition (%) |
S. No. Parameter Composition (%) |
|
1 Fixed Carbon (%) 76.2 |
1 Fixed Carbon (%) 76.2 |
1 Fixed Carbon (%) 76.2 |
|
2 Volatile Matter (%) 15.4 |
2 Volatile Matter (%) 15.4 |
2 Volatile Matter (%) 15.4 |
|
3 Ash Content (%) 6.3 |
3 Ash Content (%) 6.3 |
3 Ash Content (%) 6.3 |
|
4 Moisture (%) 2.1 |
4 Moisture (%) 2.1 |
4 Moisture (%) 2.1 |
Adsorption process
The iron-contaminated water (10.0 ppm) was prepared by adding calculated amount of FeSO4 in double distilled water. The characteristics of water taken for the study are given in the following table.
Table 2: Characteristics of the water
|
S. No. Parameter Value |
S. No. Parameter Value |
S. No. Parameter Value |
|
1 pH 6.35 |
1 pH 6.35 |
1 pH 6.35 |
|
2 Conductivity (µmho) 178 |
2 Conductivity (µmho) 178 |
2 Conductivity (µmho) 178 |
|
3 Turbidity (NTU) 6 |
3 Turbidity (NTU) 6 |
3 Turbidity (NTU) 6 |
|
4 Fe (ppm) 10.0 |
4 Fe (ppm) 10.0 |
4 Fe (ppm) 10.0 |
100 ml of the water is taken in a ground joined stoppered conical flask and mixed with required amount of coconut shell charcoal (viz.100 ppm, 200ppm….) of particle size 0.4 mm. The flask is stirred for definite time (5 min) using Red Devil shaker. Then the flask is allowed to stand still. After a regular intervals of time, the water is filtered using Whatman No. 41 filter paper and the iron present in the water is determined by colorimetric methods using Elico -SL 159 UV- spectrophotometer. The plots were drawn with iron concentrations in water against amount of charcoal.
Variation of adsorption of iron with residence time
The experimental setup is same as above.The only modification in the experiment is giving different residence time (1 hr, 2hr, 3hr, and 4 hr) for the adsorbent to remove iron from solution. The aim of the study was to optimize the residence time for the removal of iron in water. The graphs were plotted with the concentration of iron in water against residence time.
Variation of adsorption of iron with shaking time
Different shaking time is given for the system (5min, 15min, 30 min) and measure the amount of iron removed from the solution. This study is conducted to optimize the proper mixing of the adsorbent and adsorbate.
Variation of particle size of adsorbent for adsorption of iron in water
The particle size of the adsorbent is varied from 0.4 mm to 0.2 mm to understand the effective size of the adsorbent for the maximum adsorption of iron from water. Different residence time was also given to ensure the maximum absorbance. The study not only aimed to reveal the maximum adsorption capacity of the charcoal but also to know the practical feasibility of the size of adsorbent that can be implemented in water system.
MnO2 modification of the adsorbent
1.0ppm of MnO2 is incorporated in to charcoal by adding the calculated amount of KMnO4 into the charcoal of particle size 0.4 mm and dried at a temperature of 60°C for 1hr. The modified coconut shell (100 ppm) is added in the water system. The shaking time is given 5 minutes. The amount of iron removed from the system with different residence time is estimated and plotted against the residence time.
Results and Discussion
Optimization of amount of charcoal for the removal of iron from water Figure 1 shows the amount of iron removed from the water by varying the amount of coconut shell charcoal (0.4 mm size) and residence
time with constant shaking time of 5 minutes.
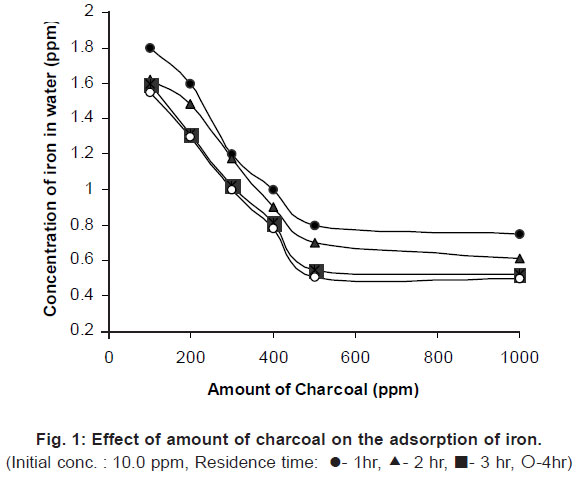 |
Figure 1: Effect of amount of charcoal on the adsorption of iron click here to view figure |
From the graph it was clear that the coconut shell charcoal was very effective to remove the iron content in the water. Increasing the concentration of charcoal and also residence time the amount of iron removal from water also increased. It was observed from the figure that the concentration above 500 ppm of charcoal can marginally improve the removal of iron from thesystem. Hence 500 ppm of coconut shell charcoal can be taken as the optimum concentration. As the residence time increases the iron removal also increases. This is due to more adsorption of iron by the adsorbent by increasing the contact time between adsorbent and adsorbate. The maximum iron removal is found by giving a residence time of 4 hrs. The decrease in the iron content in water above 3 hrs residence time was very low and hence the study would not focused to give attention for more than 4 hrs of residence time. The concentration of iron is reduced from 10.0 ppm to 0.5 ppm by adding 1000 ppm coconut shell charcoal having a residence time of 4 hours. This value lies above the standard value (0.3 ppm). So further study focused to increase the shaking time of the system.
Effect of shaking time for the removal of iron in water
The shaking time was increased from 5 minutes to 30 minutes and noted the amount of iron removal at various residence time. The graph is plotted by the amount of iron against the shaking time and is given below.
 |
As the shaking time increases the iron removal also increases. This is due to proper intermixing of adsorbate and adsorbent. But increasing the shaking time has less importance since it is very difficult to implement in practical environments. The minimum iron content in the system was found to be 0.5 ppm using 500 ppm coconut shell charcoal at a shaking time of 30 minutes and a residence time of 4 hrs.
Behaviour of adsorption of iron with respect to particle size of the coconut shell charcoal
The adsorption behaviour of iron with respect to the particle size of the coconut shell charcoal is given in Fig. 3.
As the particle size decreases the amount of iron get adsorbed on coconut shell charcoal from the water increases. This is due to more effective surface area of the adsorbent for adsorbing the iron.
The iron concentration was bring down to the standard value of 0.3 ppm in water by using 500 ppm coconut shell charcoal having a size of 0.2 mm and giving a residence time of 4 hrs without increasing the shaking time beyond 5 minutes. A problem was noticed while using the coconut shell charcoal of having 0.2 mm is that it is very difficult to settle down the adsorbent and to remove it from the system. Hence it is found to be practically not feasible to reduce the particle size below 0.4 mm.
Effect of MnO2 incorporated charcoal on adsorption of iron
MnO2 modified charcoal has been introduced into the system and the removal of iron was estimated and compared with bare charcoal water system. The results of the study are given in Fig. 4.
 |
Figure 3: Variation of adsorption of iron with particle size of coconut shell charcoal click here to view figure |
The iron concentration in water was found to be decreased to the level of 0.2 ppm by using the MnO2 modified coconut shell charcoal at a residence time of 4 hrs. The increase in the iron removal is due to the increase in the effective adsorption sites by the incorporated MnO2 particles and the catalytic effect of these MnO2 particles tooxidize Fe2+ to Fe3+. The Fe3+ precipitates on the solid, forming a hydrated iron oxide coated carbon.
 |
Figure 4: Effect of MnO2 incorporated charcoal for iron removal click here to view figure |
Conclusion
- The coconut shell charcoal has been found to be a good adsorbent to remove the iron in drinking water and optimum concentration was found to be 500ppm.
- As the particle size of coconut shell charcoal decreases, the iron removal increases. But the particle size beyond 0.4 mm cannot be recommended due to difficulty of removing the adsorbent from the system.
- As the shaking time increased the adsorption also get increased. Here also more shaking time cannot be recommended due to the limitation of applying in practical condition
- The optimum residence time for the removal of iron in water was found to be 4 hours
- MnO2 incorporated coconut shell charcoal is very effective to remove the iron content in water below 0.3 ppm without increasing the pH.
References
- J. D. Navratil., M.T. S. Tsair, Magnetic separation of iron and heavy metals fromwater, Water science and Technology. 2002;47:29-32.
- Guidelines for Drinking water Quality, Recommendations, W.H.O., Vol .1, Geneva, Switzerland 79. 1984.
- Das B., P. Hazarika., G. Saikia., H. Kalita., D.C. Goswami., H.B. Das., S.N. Dube., R. K. Dutta. Removal of iron from groundwater by ash: A systematic study of a traditional method, Journal of Hazardous materials. 2007;141:834-841.
- Ryoma Bun- E1., N. Kawasaki., F. Ogata., T. Nakamura., K. Aochi., S. Tanada. Removal of lead and iron ions by vegetable biomass in drinking water, Journal of oleo science. 2006;55:423-427.
- P. Q. Governor., D. T. Arnold. Llinois Department of Public Health http://www.idph.state.il.us/envhealth/factsheets/iron.
- C. N. Sawyer, et.al. Chemistry for Environmental Engineering and Science, Fifth edition by Tata McGraw - Hill. 2003;659-665.
- K. Vaaramaa and J. Lehto, Removal of metals and anions from drinking water by ion exchange, Desalination. 2003;155:157-170.
- D. Ellis., C. Bouchard., G. Lantagne. Removal of iron and manganese from ground water by oxidation and microfiltration,Desalination. 2000;130:255-264.
- A. P. Ralph. Apparatus for removal of iron from drinking water, US Patent No. 5,180,491 dated 19 January 1993.
- W.C. Andersen., T. J. Bruno. Application of gas- Liquid entraining rotor to supercritical fluid extraction removal of iron (iii) from water, Analytica chimica acta. 2003;485:1-8.
- P. Berbenni., A. Pollice R. Canziani, L. Stabile and F. Nobili Removal of iron and manganese from hydrocarbon - contaminated ground waters, Bioresources Technology. 2000;74:109-114.
- H. A. Aziz. Physicochemical removal of iron from semiaerobic landfill leachable by limestone filter, Water Management. 2004;24:353-358.
- J. H. Potgieter, et.al. Removal of iron and manganese from water with a high organic carbon loading, Part I The effect of various coagulants, http://cat.inist.fr/ A modele =affichen & cpsidt = 1685564.
- R. Munter., H. Ojaste., J. Sutt. Complexed iron removal from ground water, Journal of Environmental Engineering. 2005;131:1014-1020.
- Regina de F.P.M. Moreira., V. S. Madeira., H.J. Jose., E. Humeres. Removal of iron from water using adsorbent carbon, Separation science and Technology. 2005;39:271-285.






