Removal of divalent manganese from water by adsorption using gac loaded with Ethylene Di-amine Tetra Acetic acid (EDTA) and Nitrilo Tri-acetic Acid (NTA)
U. E. Chaudhari1 *
DOI: http://dx.doi.org/10.12944/CWE.4.2.24
The studies on removal of Manganese (II) were conducted using Granulated Activated Carbon (GAC) in combination with Chelating agents, Ethylene di-aminetetraacetic Aced (EDTA) and Nitrilotriacetic Acid (NTA) at 25°C. The adsorption efficiency was studied in the pH range. The results reveals that Langmuir and Freundlich isotherms are followed during adsorption process with the chelating agents under study. The granulated activated carbon (GAC) loaded with Ethylene di-aminetetraacetic acid (EDTA) shows greater adsorptive capacity as compared to granulated activated carbon (GAC) loaded with the chelating agent. Nitrilotriacetic acid (NTA).
Copy the following to cite this article:
Chaudhari U.E. Removal of divalent manganese from water by adsorption using gac loaded with Ethylene Di-amine Tetra Acetic acid (EDTA) and Nitrilo Tri-acetic Acid (NTA). Curr World Environ 2009;4(2):413-417 DOI:http://dx.doi.org/10.12944/CWE.4.2.24
Copy the following to cite this URL:
Chaudhari U.E. Removal of divalent manganese from water by adsorption using gac loaded with Ethylene Di-amine Tetra Acetic acid (EDTA) and Nitrilo Tri-acetic Acid (NTA). Curr World Environ 2009;4(2):413-417. Available from: http://www.cwejournal.org/?p=100
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2009-08-22 |
|---|---|
| Accepted: | 2009-09-30 |
Introduction
Water is a Prime natural resource and is a basic human need. It is available in nature as surface water and ground water through the self purification mechanisms like physical, chemical and microbiological processes at natural bodies are carried out in nature. However, natural water is rarely suitable for direct consumption to human beings. Rapid industrialization and population growth resulted to generation of large quantities of wastewater and causing problem of their disposal. Industrial waste constitutes the major source of various kinds of metal pollution in natural water. The presence of heavy metals in the environment has been of great concern because of their increased discharge, toxic nature and other adverse effects on the receiving streams. When the concentration of toxic metal ions exceed tolerance limit. They may become real health concern1. There is an immediate need to introduce cleaner technologies to minimise the pollution and to protect the degrading environment. It is not possible to achieve zero waste discharge, but it is an essential to treat the waste.
Among the toxic heavy metal ion which present in potential health hazard to aquatic animals and human life, Pb, Cd, Cr, V, Bi and Mn are important. Toxicity of manganese (II) and its salt include Psychological disorders, Neurological disorder, Manganese Pneumonia, Bronchitis, nose and throat infection increased respiration, hypo- sexuality, tremor of the fingers, muscular rigidity, chronic bronchitis and decrease liver activity. Manganese ethylene-bis-dithiocarbomide is found to be carcinogenic and cause cancer. Divalent manganese has been found to be 2.5 to 3 times more toxic than trivalent form.
Literature survey reveals that, there are many methods namely coagulation, precipitation, ion exchange and adsor ption for removal of manganese metal ions from aqueous medium. However, adsorption is an easy and economical process for removal and retrieval of cation from aqueous medium. Efficiency of adsorption process mainly depends on nature of adsorbent, adsorbate, pH, concentration, temperature, time of agitation etc. There is a need to enhance the adsorptive power of an adsorbant by using the same with various type of chelating agents. These cheap and efficient adsorpbents can carry to cater the need of population in the rural areas and the population in the industrial area where safe drinking water is not available. In the present study, and attempt has been made to increase the adsorption efficiency of G.A.C. by using chelating agents namely EDTA and NTA.
Materials and Methods
Preparation of Adsorbents (Granuler Activated Carbon)
The commercially available activated Carbon was selected as an adsorbant for the present study. The carbon was sieved through a mesh size (- 12 X 18) (M/S Jayant Test sieves, Mumbai) and washed with distilled water, several times until the leachate was free from any suspended impurities. The washed sample was then dried in an oven at 100-110°C and stored in a calcium chloride desiccator until use.
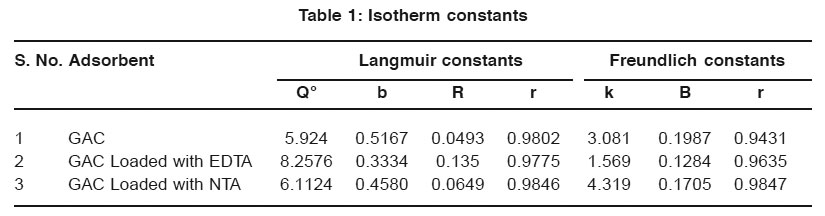 |
Table 1: Isotherm constants Click here to view table |
Batch Study
The dried amount of 0.5 gms of GAC was taken in 1000 ml in round bottom flask and synthetic solution (200ml) containing various concentrations of various amount of manganese (II) ion was added and was stirred using remistirrer at 1000 r.p.m. in a thermostat maintained at (25+1)° C for six hours.
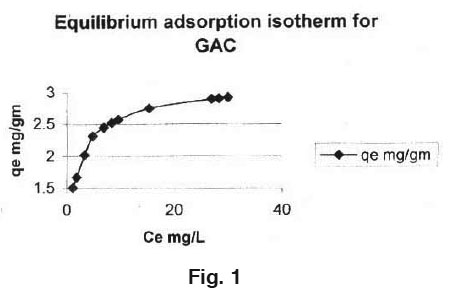 |
Figure 1 Click here to view figure |
Similarly 0.5 gms. of GAC was taken in shaking bottle and 100 ml of 0.002 M of ligand (EDTA or NTA) was adsorbed on it. This process of fixing a ligand (EDTA or NTA on GAC is denoted as "Loading of GAC". Then the bottles were shaken at room temperature (25+1)°C. Once the loading of GAC with ligand (EDTA or NTA) was done, synthetic solution (200 ml) containing various concentrations of Manganese ions was added to the loaded activated carbon in 1000 ml, round bottom flask, and stirred using remistirrer at 1000 r.p.m. in thermostat maintained at temp (25+1)oC, for six hours.
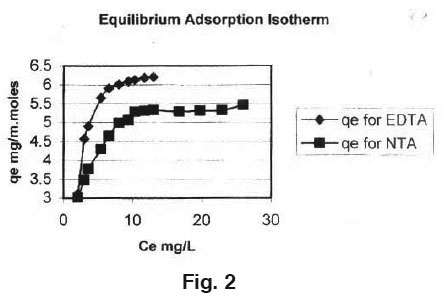 |
Figure 2 Click here to view figure |
The initial and final concentrations of Manganese (II) metal ion was determined by spectrophotometry³ using periodate method and measured absorbance at 460 nanometer. The spectrophotometer, Systronic (model 104) was used to measure the concentration of Manganese (II) ions.
 |
Figure 3 Click here to view figure |
Results and Discussions
Equilibrium adsor ption isother ms for Ce Vs qe plotted for activated carbon are shown in Fig. 1. The adsor ption capacity in mg/L was calculated from the equation.
qe = (Co-Ce)V/M
where,Co is the initial concentration of Manganese (II) Ce is the concentration of Manganese (II) at equilibrium in mg/L V is the volume of solution in litre and M is the mass of adsorbent in grams
Similarly equilibrium adsorption isotherm for Ce Vs qe plotted for EDTA and NTA adsorbed on GAC is shown in Fig. 2 The adsorption capacity was calculated from the equation:
qe = (Co-Ce)V/M
where,
qe represents the maximum amount of Manganese that millimole of EDTA and NTA can hold.
Co = initial concentration of Manganese ion in solution in mg/L
Ce = concentration of Manganese ion at equilibrium in solution in mg/L
V = Volume of solution in litre and
W = Millimole of ligand (EDTA or NTA)
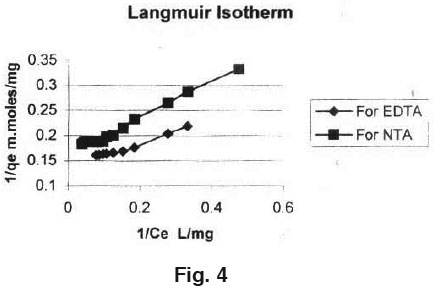 |
Figure 4 Click here to view figure |
Adsorption Isotherms
Equilibrium isotherms was studied for both Langmuir and Freundlich isotherms. The results are shown in Fig. 3, 4 and 5 which, illustrate the plot of Langmuir and Freundlich isotherms of activated carbon loaded with EDTA and NTA for Manganese. The saturated monolayer can be represented by:
qe = Q0.b.Ce 1+b.Ce
The linearised from of the Langmuir isotherm is 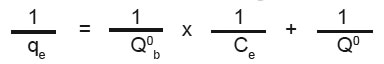 Where, Q0 and b are Langmuir constants.
Where, Q0 and b are Langmuir constants.
The plot of 1/Ce Vs 1/qe was found to be linear, indicating the applicability of Langmuir model. The parameters Q0 and b have been calculated and presented in Table 1. The Langmuir constant Q0 is a Measure of adsor ption capacity and b is a measure of energy of adsorption. In order to observe whether the adsorption is a favourable or not, a dimensionless parameter 'R' obtained from Langmuir isotherm is R = (1 + b x Cm)-1​​​​​​​ Where, b is Langmuir constant and Cm is maximum concentration used in the Langmuir isotherm.
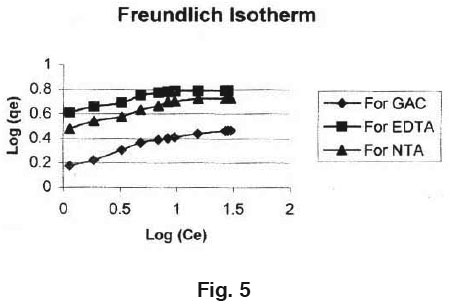 |
Figure 5 Click here to view figure |
The adsorption of Manganese on GAC loaded with ligand is a favourable process as 'R' values lie between zero to one. Coefficients of co- relation (r) are also shown in Table 1. The applicability of Freundlich isotherm was also tried using the following general equation: qe = k.Ce B linearised form of this equation is log qe = B.log Ce+ log k. Where, B and k are Freundlich constants.
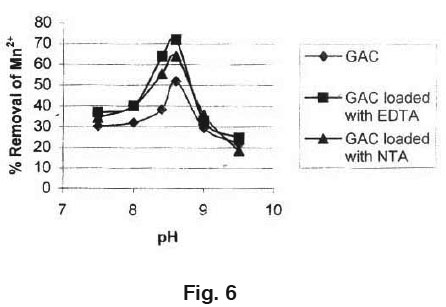 |
Figure 6 Click here to view figure |
These constants represent the adsorption capacity and the adsorption intensity respectively. Plot of log qe Vs log Ce was also found to be linear.The values of B and k are presented in Table 1. Since the values of B are less than 1, it indicates favourable adsorption.
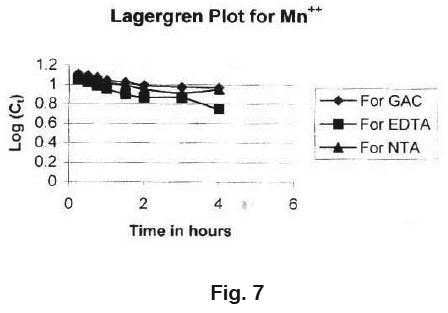 |
Figure 7 Click here to view figure |
Effect of pH on the Removal of Manganese (II)
The effect of pH on the removal of Manganese (II) is shown in Fig. 6 Experiment were conducted at the constant initial Manganese (II) concentration, adsorbent dose (GAC) of 0.5 gm/100 ml and the contact time of 3 hours. Results indicate that GAC loaded with EDTA has the maximum adsor ption capacity for removal of Manganese (II) as compared to GAC loaded with NTA and GAC used independently.
This establishes that the pH of the aqueous solution is an important controlling parameter in the adsorption process. It was obser ved that the percentage removal of Manganese is higher at pH= 8.6 and then decreases with increase of pH.
Kinetics of Adsorption
The kinetics modelling for the removal of Mn2+ by GAC, GAC loading with EDTA and NTA has been carried out and rate constant for adsorption was determined by using lagergren equation. log Ct = log Co - 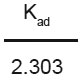 x t
x t
The straight line plots of log C versus time for the adsorption show the validity of lagergren equation suggest the first order kinetics (Fig. 7)
- Removal of Manganese (II) by adsorption with GAC loaded with ligand (EDTA or NTA) has move adsorptive power as compared to the use of GAC alone.
- The developed technique of retrieval of Manganese (II) ions using GAC loaded with ligand appears to be a cheap and practically viable for the use of semiskilled workers in villages.
- The present work on adsorption process is in good agreement with Langmuir isotherm indicating monolayer adsorption process.
- The results on adsorption process reveals that at pH = 8.6, Manganese (II) uptake capacity is better.
- The straight line plots of log C versus time for the adsor ption show the validity of Lagergren equation for the adsorption of Mn2+ on GAC, GAC loaded with EDTA and NTA which suggest the first order kinetics.
References
- Singh D. K. and Lla Jyosna, "Removal of toxic heavy metal ion from wastewater by coal based adsorbent", Pollution Research, (1992). 11: 37-42
- N. IRVING. SAG. "Dangerous properties of Industrial materials "Published by Van Nost and Reinhold Company Ine. New York (1984).
- Vogel, A. I. "Textbook of quantitative Inorganic Analysis". Fourth Edition, (1978). 735-736






