Quality parameters of ground waters in Borsad and Anklav taluka (Dist: Anand, Gujarat)
Shailesh H. Shah1 *
1
Patel J.D.K. Davolwala Science College,
Borsad,
388540
India
DOI: http://dx.doi.org/10.12944/CWE.4.2.14
The present study deals with the Quality Parameters of Ground waters of Borsad and Anklav taluka village of Anand district of Gujarat state of India. The Ground water quality was assessed by examing various Physico-chemical parameters. Twenty-eight ground water (Borewell) samples were collected from different villages of Borsad and Anklav taluka during the month of May-2008, September-2008, April-2009 and August-2009. The Physico-chemical parameters like Temperature, PH, TDS, DO, Total-hardness, Ca-hardness, Mg-hardness, Total alkalinity, Chloride, Sulphate, Nitrate and Phosphate have been analyzed. In the light of above results the ground water of Borsad and Anklav taluka villages, some villages are not suitable for drinking purpose. The ground water must be subjected to proper disinfect ion to ensure health of population.
Copy the following to cite this article:
Shah S.H. Quality parameters of ground waters in Borsad and Anklav taluka (Dist: Anand, Gujarat). Curr World Environ 2009;4(2):359-365 DOI:http://dx.doi.org/10.12944/CWE.4.2.14
Copy the following to cite this URL:
Shah S.H. Quality parameters of ground waters in Borsad and Anklav taluka (Dist: Anand, Gujarat). Curr World Environ 2009;4(2):359-365. Available from: http://www.cwejournal.org/?p=983
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2009-09-10 |
|---|---|
| Accepted: | 2009-10-13 |
Introduction
The portion of the water seeping into soil in excess of that held as film surrounding soil particles continues its downward passage until it reaches an impervious stratum at which point it tends to accumulate. The geological stratum super imposed upon the impervious layer then becomes saturated, and is known as the zone of saturation. The upper level of the saturated stratum is called the water table. The depth of the water table below the surface varies widely. A formation, which will yield, stored water to wells or springs is called an aquifer. Water thus accumulating constitutes the stored ground source that assumes an important role in providing for domestic water needs.
Groundwater is an important part of the water cycle. Ground water accounts for more than 80% of the rural domestic water supply in India data collected in 1998 for the 54th round of the National Sample sur vey showed that 50% of rural households were served by a tubewell/handpump, 26% by a well and 19% by tap. Most of Indian towns and cities do not have access to safe drinking water. In most parts of the country, the water supplied through groundwater is beset with problems of quality.
In most of the villages borewells water is used for dr inking pur pose another domestic purpose. Borewells water is the under ground water that has come mainly from the seepage of surface water and is held subsoil and previous rocks. Borewells water is generally good quality and it is difficult to pollute borewells water. The use of fertilizers, pesticides and insecticides in rural area, manure, lime, septic tank, refuse dumps etc are the main source of borewells water pollution.
Materials and Methods
Twenty-eight samples of Ground water (Bore well) were collected from different villages of Borsad and Anklav taluka of Anand district of Gujarat state with few interior places in the month of May-2008, September-2008, April-2009 and August-2009 have been tested. Samples for analysis with standard procedure in accordance with standard method of Amer ican Public Health Association (APHA-1998). The Instruments were used in the limit of precise accuracy and chemicals used were of GR grade. Temperature, PH and TDS were measured using appropriate instruments. The Total hardness, calcium and magnesium concentration in the ground water samples were measured by titrimetric methods. The total hardness was determined by titrating the buffered water sample with 0.01M EDTA. The calcium hardness was deter mined by adding calcium hardness indicator, 1ml of 8% NaOH into a sample of water, mixing thoroughly and titrating with EDTA to give a pur ple colour. The magnesium hardness was obtained from the difference between total hardness and calcium hardness. The chloride content was determined using silver nitrate titrant and potassium chromate as the indicator under neutral conditions. The dissolved oxygen was measured using Winkler method. The Total alkalinity was determined by titrimetric methods using phenolphthalein and methyl orange indicators. The Sulphate, Nitrate and phosphate were measured using the spectrophotometer.
Results and Discussion
Temperature
It is one of the most essential parameters in water and waste water system. It has significant impact on growth and activity of ecological life and it greatly affects the solubility of such as oxygen in water. Oxygen levels have decreased, as the temperature tends to increase the molecular motion of the water and any dissolved oxygen. The temperature of the ground water samples of different villages of Borsad and Anklav taluka of Anand district were found to be in the range 28° c to 38° c the measured temperature were reported in table.
pH
pH value is the best indication of the presence of acid or alkali in water samples. PH of ground water of Borsad and Anklav taluka villages va r ies from 7.2 to 8.7. The acceptable limit prescribed by the drinking water standard is 6.5-8.5.
Dissolved Oxygen
D. O. is one of the most impor tant parameters in assessing water quality and reflects the physical and biological processes prevailing in the water. Good water should have the solubility of oxygen 7.6 and 7.0 mg/l at 30°c and 35°c respectively. Oxygen saturated water have pleasant taste. In the present study of the D.O. values of Ground water samples ranged from 1.4 mg/l to 7.4 mg/l, According to the European Economic Community, the permissible standard for drinking water for DO is 5 mg/l. As per the ISI the minimum dissolved oxygen recommended is 3 mg/l. In the present study, DO Values of sample station Nos. 1,2,5,7,8,10,12,14,16,17,18,21,24,25,26 &27 show lower DO than prescribed by ISI. Hence remaining sample station (villages) is not polluted with respect to dissolved oxygen.
Total Dissolve Solids (TDS)
TDS is an important parameter for Drinking water and water to be used for other purposes. The upper limit of TDS recommended for Drinking water is 500 mg/l by USEPA (1996), 1000 mg/l by WHO (1993) and the permissible limit in the absence of alternate source is 2000 mg/l by IS: 10500(1991). In the present study TDS ranged from 390 mg/l to 1650 mg/l.
Total alkalinity
Alkalinity is the quantitative capacity of an aqueous media to react with hydrogen ions. Desirable limit is 200 mg/l and maximum permissible limit 600 mg/l. In the present study total alkalinity ranged from 350 mg/l to 828 mg/l. so, sample station Nos.1, 2,3,4,6,7,8,17,23,26 & 27 show higher than prescribed by IS: 10500.
Chloride
Chlorides are common constituents of all natural waters. Higher concentrations of chloride impart a salty taste to water, making it unacceptable for public consumption. As per the Bureau of Indian Standards the desirable limit of chloride for Drinking water is 250 mg/l and the permissible limit in the absence of alternate source is 1000 mg/l. The chloride values of Ground water vary from 63.8 mg/ l to 843.9mg/l. All the ground water samples showed chloride value within a permissible limit.
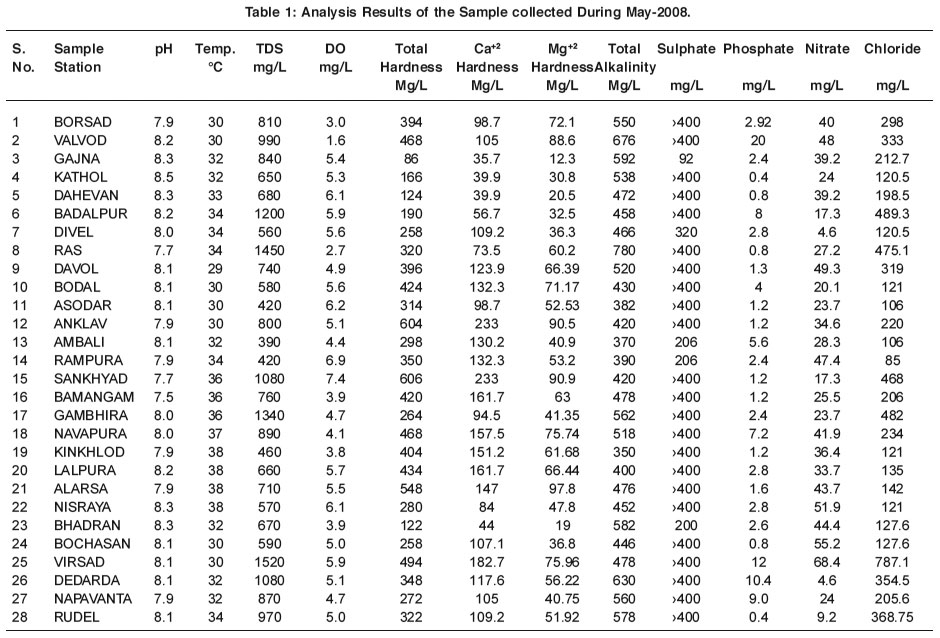 |
Table 1: Analysis Results of the Sample collected During May-2008. Click here to view table |
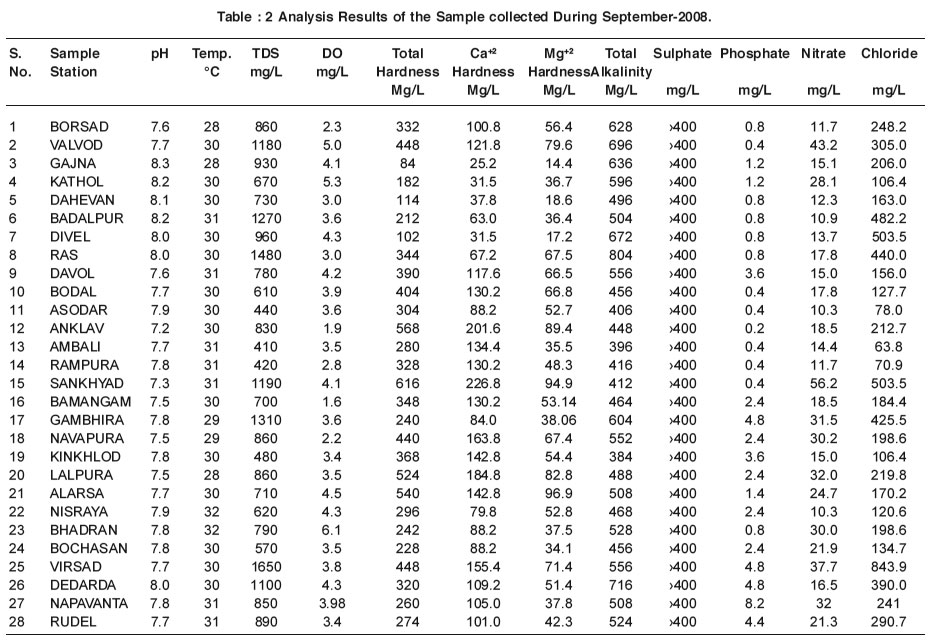 |
Table : 2 Analysis Results of the Sample collected During September-2008. Click here to view table |
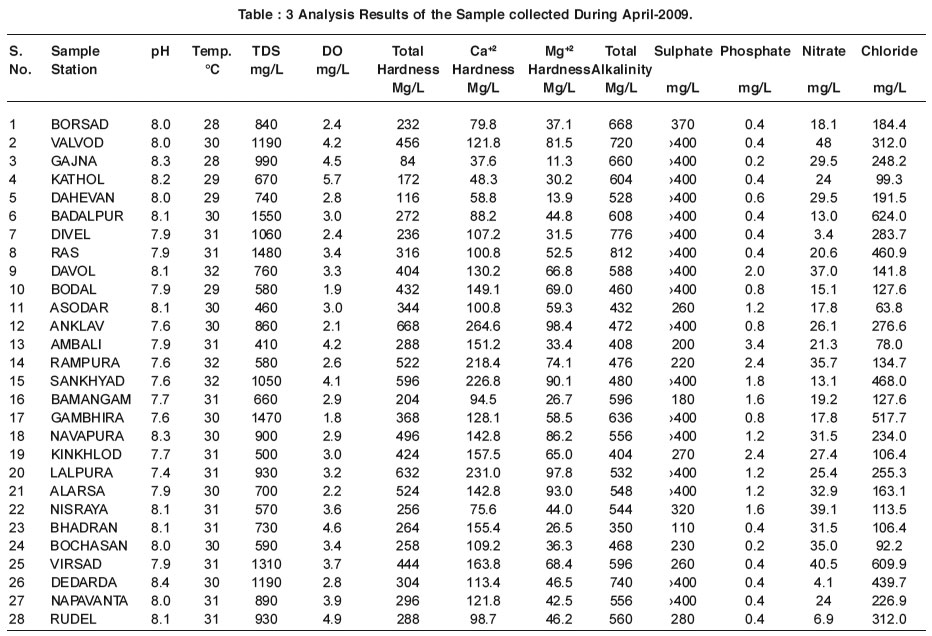 |
Table : 3 Analysis Results of the Sample collected During April-2009. Click here to view table |
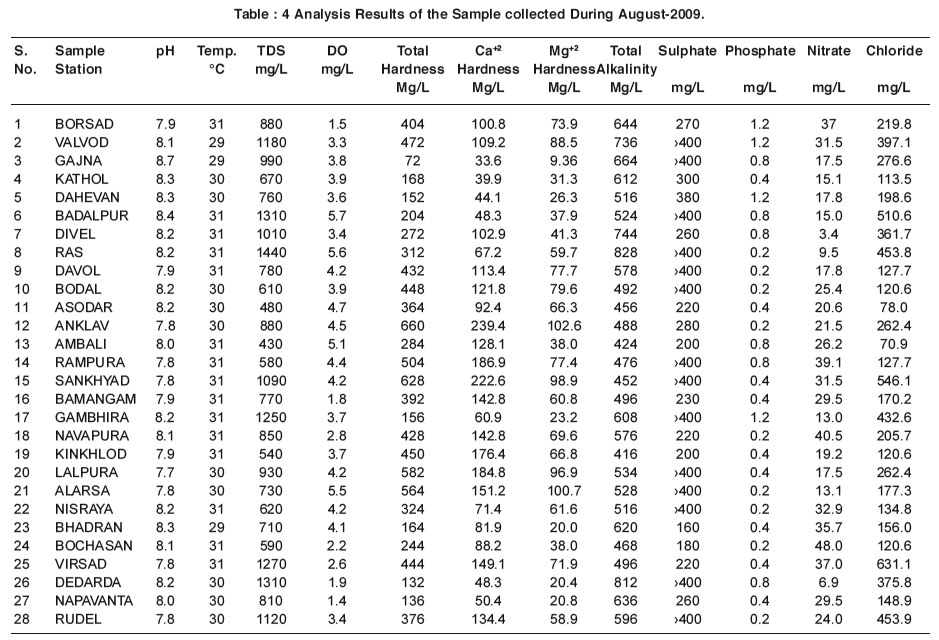 |
Table : 4 Analysis Results of the Sample collected During August-2009. Click here to view table |
Total Hardness
Water hardness is caused by the presence of calcium and magnesium salts. Hardness of ground water samples varies from 72 mg/l to 668 mg/l. As per the IS: 10500 the desirable limit of total hardness for Drinking water is 300 mg/l and the permissible limit in the absence of alternate source is 600 mg/l.
In the present study Total Hardness values of sample station Nos. 1,2,8,9,10,11,12, 14,15,16,17,18,19,20,21,22,25,26, & 28 were beyond desirable limit while sample station Nos. 12,14,15 and 20 are beyond permissible limit in the absence of alternate source. Water with hardness more than 150 mg/l is found to be objectionable for domestic purpose. In the present study Ca-Hardness values of sample station Nos. 12,14,15 & 20 were above permissible limit of 200 mg/l. Mg-Hardness values of sample station Nos.12 and 21 were above permissible limit of 100 mg/l.
Sulphate
Presence of Sulphate has less effect on the taste of water compared to the presence of chloride. The desirable limit of Sulphate in drinking water prescribed by ICMR is 200-400 mg/l. In the present study Sulphate ranged 92 mg/l to greater than 400mg/l. The high concentration of Sulphate may induce diarrhea and intestinal disorders.
Phosphate
It is an essential nutrient element for growth, propagation and activity of plants, animals, even aquatic species and microbes. Phosphate in water occurs in the for m of or thophosphate, polyphosphate and organic phosphate. The values of phosphate concentration of different village samples vary from 0.2 mg/l to 20 mg/l.
Nitrate
The concentration of different forms of nitrogen give a useful indication of the level of micronutrients in the water and hence their ability to support plant growth. A high content of NO3- N in water may be toxic to babies when used for making up feeds from milk powders. The observed levels of nitrate concentration, 3.4 mg/l to 68.4 mg/ l. In the present study sample station Nos. 15, 22, 24 and 25 shows higher than prescribed by WHO upper limit of 50 mg/l for domestic water.
Acknowledgements
Author are thankful to the UGC for financial assistance in the form of Minor Research Project {F.No.47-103/07 (WRO)}, Author are also thankful to my students Hiren Patel, Tarang Prajapati, Riken Patel and also thankful to Patel JDK Davolwala Education Trust, Borsad and the Principal of Patel JB Rudelwala Arts, Patel AM Rudelwala Commerce & Patel JDK Davolwala Science College, Borsad for providing necessary facilities.
References
- Standard Methods for Examination of Water and Waste water,20th Edn., APHA,AWWA AND WPCF, Inc., New York (1998).
- WHO,(Wor ld Health Organisation) Guidelines for Dr inking Water Quality, Recommendations, WHO Geneva (1993).
- V.P.Kudesia, water pollution, Pragati Prakashan, Meerut (1985).
- Maiti S. K., hand Book of Methods in Environmental Studies. 1 water and waste water analysis 1st edition, ABD Publishers, Jaipur (2004).
- De. A. K., Environmental Chemistry, 5th edition, New Age Int. Ltd. Publishers, New Delhi (2004).
- R.K. Trivedi, P.K. Goel & C.L. Trisal, Practical Methods in Ecology And Environmental Science, Enviro Media Publications, KARAD (India), (1998).
- Saxena M. M., Environmental Analysis Water, Soil and Air, Agro Botanica Publication, Bikaner (1998).






