Quality of drinking (surface and sub-surface) water in relation to human health of Sehore town, M.P. (India)
Rachna Sharma1 * , Anand Sharma2 and D.R. Tiwari3
1
Department of Chemistry,
LNCT,
Bhopal,
462 001
India
2
Department of Chemistry,
MVM,
Bhopal,
462 001
India
3
Department of Geology,
MVM,
Bhopal,
462 001
India
DOI: http://dx.doi.org/10.12944/CWE.4.2.30
Water is most essential to life next to air only. Surface water and Sub-surface water both are very important for water supply for irrigation, industries and drinking purpose. Safe drinking water is primary needs of every person. Most of person mainly depends upon ground water sources. Some of these have problems such as excess hardness, Sodium, Potassium, Calcium, Nitrate etc. The natural quality of surface water and Sub-surface water tends to be degraded by human activities and geo environmental changes. Physico-chemical analysis of rivers and bore well and hand pumps has been collected in pre-monsoon (Aug-08) and Post-monsoon (Dec-08). Different parameter of water has been analyzed and assessed the suitability of drinking water in public hygiene scenario. Some parameters are with in the range as prescribed by ISI and WHO while other are beyond the limits.
Copy the following to cite this article:
Sharma R, Sharma A, Tiwari D.R. Quality of drinking (surface and sub-surface) water in relation to human health of Sehore town, M.P. (India). Curr World Environ 2009;4(2):435-438 DOI:http://dx.doi.org/10.12944/CWE.4.2.30
Copy the following to cite this URL:
Sharma R, Sharma A, Tiwari D.R. Quality of drinking (surface and sub-surface) water in relation to human health of Sehore town, M.P. (India). Curr World Environ 2009;4(2):435-438. Available from: http://www.cwejournal.org/?p=1014
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2009-08-30 |
|---|---|
| Accepted: | 2009-10-27 |
Introduction
Now a day's ground water is primar y sources of drinking water. The quality of ground water depends on the ion, which are dissolved in ground water. The major ions which are responsible to maintain the quality of ground water are carbonate, bicarbonate, chloride, sulphate, nitrate etc. These ions are present in ionic form. The cation and anions must be equal to maintain the quality of water. Cation such as calcium ion, magnesium ion, sodium, potassium etc. are also present in ground water in the form of hardness and salinity. The natural quality of ground water tends to be degraded by human activities. Municipal and Industrial water entering into an aquifer are the major sources of organic and inorganic pollutants. The water level of underground water has been full down to 30-40 meters in most of areas. Eight surface water samples and eight Sub-surface water samples collected from Sehore town and they ware analyzed for parameters such as pH, total alkalinity, total hardness, and calcium ions, magnesium ion, Na+, K+, NO3- etc.
Material and Methods
Water samples of various hand pumps and tube wells from eight stations were collected in polythene bottles as per the standard procedure and were transpor ted to laboratory for various chemical analysis. The physico-chemical parameters such as pH, Temperature, Turbidity, Total Alkalinity (TA), Total hardness (TH), Calcium (Ca2+), Magnesium (Mg2+), Sodium (Na+), Potassium (K+), Chloride (Cl-), Sulphate (SO2-), Nitrate (NO3), were determined 4 3 quality of ground water tends to be degraded by human activities. Municipal and Industrial water entering into an aquifer are the major sources of organic and inorganic pollutants. The water level of underground water has been full down to 30-40 meters in most of areas. Eight surface water samples and eight Sub-surface water samples collected from Sehore town and they ware analyzed for parameters such as pH, total alkalinity, total hardness, and calcium ions, magnesium ion, Na+, K+, NO3- etc. using standard method Reagents used for the present investigation were AR grade and double distilled water was used for preparing various solution.
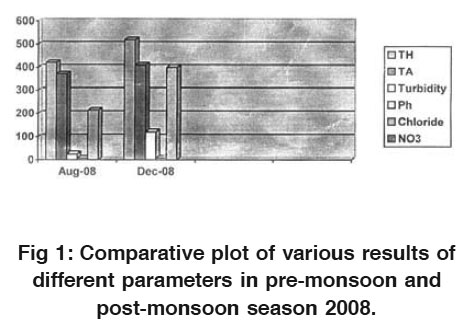 |
Figure 1: Comparative plot of various results of different parameters in pre-monsoon and post-monsoon season 2008. Click here to view figure |
 |
Figure 2: Comparative plot of various results of different parameters in pre-monsoon and post-monsoon season 2008. Click here to view figure |
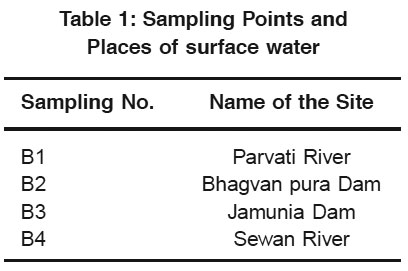 |
Table 1: Sampling Points and Places of surface water Click here to view table |
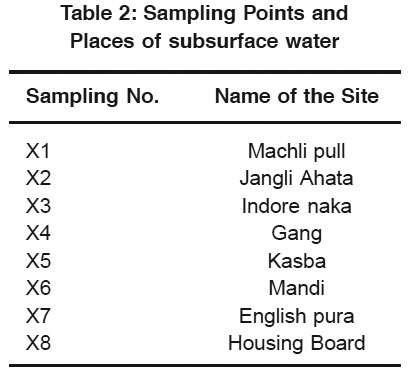 |
Table 2: Sampling Points and Places of subsurface water Click here to view table |
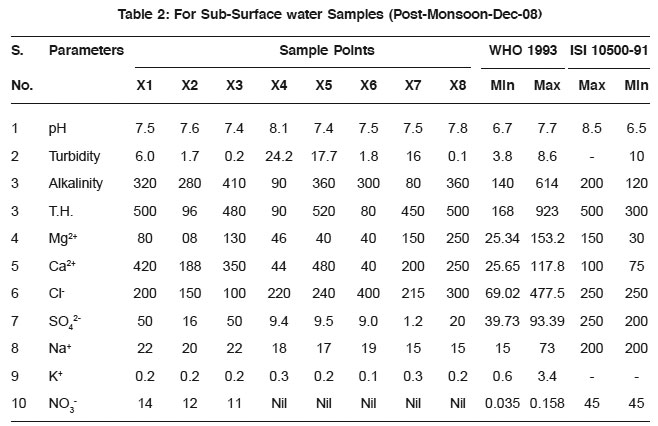 |
Table 2: For Sub-Surface water Samples (Post-Monsoon-Dec-08) Click here to view table |
Results and Discussion
The average values of physicochemical parameters during Aug-08 and December 2008 are presented in Table 1, 2, 3, and 4. Determination of chemical characteristics is essential for assessing the suitability of water drinking, industrial and household uses.
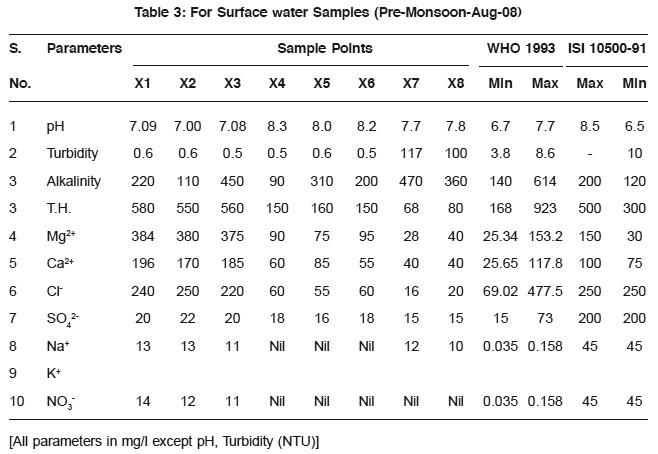 |
Table 3: For Surface water Samples (Pre-Monsoon-Aug-08) Click here to view table |
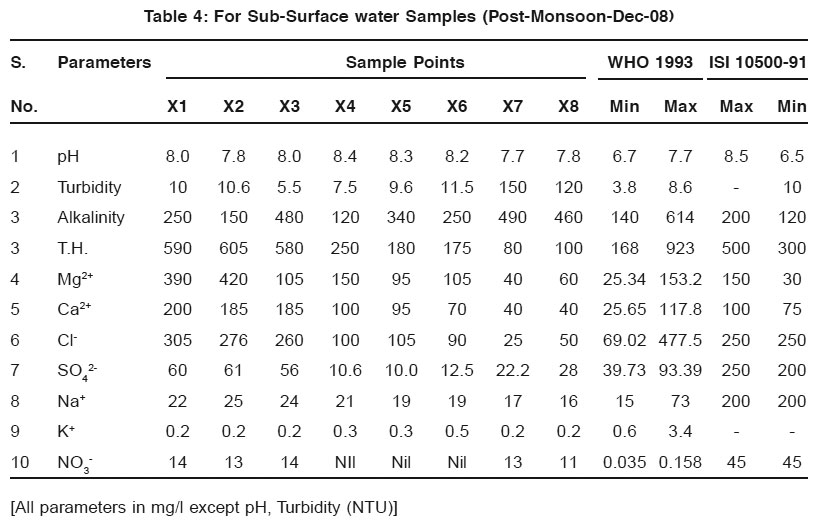 |
Table 4: For Sub-Surface water Samples (Post-Monsoon-Dec-08) Click here to view table |
The observed pH values show that almost all the water samples are in permissible limit as prescribed by WHO.
Turbidity is higher/greater in samples taken in post monsoon season then those of pre-monsoon samples. The possible reason may be the depletion of groundwater level.lkalinity also increases along with all other parameters and these are shown in the Fig. 1 and Fig. 2.
References
- WHO, International standard for drinking water Genera (1984).
- APHA standard method for examinations of water and waste water (19th Edn.) American Public Health Association, Washington, OC (1995).
- India standard specification for Drinking Water, ISI, New Delhi (1991).
- ICMR, Manual Standards of Water Quality for Drinking Water supplies, 2nd Edition, Indian Council of Medicinal Research, New Delhi 21 (1973).
- Holden, W. S. Water Treatment and Examination church hill London pp 513 (1970).
- Rebhun M., Goodman N. 2. and Barker F. B., J. Water Pollution Control Fed.,(1987) 59: 242-248.
- Anand Sharma and Tiwari D. R.,(2008) Vol 3(1): 199-202.
- B. K. Sharma, Environmental Chemistry, Sixth Edition, 2001 (water 17-18).
- Hando, B. K. Ground water Contamination in India, Proc. Regional Wo r kshop on Environmental aspects of Groundwater development, Kurukshetra,(1994) 1-13.
- NEERI: Manual on water and waste water analysis, National Environmental Engineering Research Institute, Nagpur 340, (1986).
- Kataria, H. C., et. al. Physicochemical analysis of water of Kubza r iver of Hoshangabad, Orient. J. Chem.,(1995) 11(2): 157-159.
- BIS: 10500: Drinking water specification, first revision, Bureau of Indian standards New Delhi (1991).
- Kataria, H. C., et. al., Physico-Chemical investigations of Dahod dam water, Orient. J. Chem.,(2004) 20(4): 703-706.






