Genotoxic effect of paper mill effluent on chromosomes of fish Channa punctatus
M.K. Malik1 * , Parmesh Kumar1 , Reenu Seth1 and S. Rishi1
DOI: http://dx.doi.org/10.12944/CWE.4.2.13
Drainage of effluent from different types of industrial units poses a serious threat to the aquatic flora and fauna. Fish being an inhabitant of the closed aquatic environment, serve as a useful model for assessing the effect of chemicals mixed in the aquatic environment. In the present study, genotoxic effect of an effluent from a paper mill located at Kurukshetra was tested in Channa punctatus, a fresh water fish. The fish were exposed to the effluent for 24, 48, 72 and 96 hours. The remarkable chromosomal aberrations observed in the treated specimens included centromeric gaps, chromatid break, attenuation, acentric fragments, pycnosis, polyploidy and chromosomal gaps. The total number of aberrations was significantly higher in treated specimens as compared to controls. The results of present study clearly points genotoxic potential of the paper mill effluent on the fish chromosomes. The effluent, therefore, should be treated before discharged into the water bodies
Copy the following to cite this article:
Malik M.K, Kumar P, Seth R, Rishi S. Genotoxic effect of paper mill effluent on chromosomes of fish Channa punctatus. Curr World Environ 2009;4(2):353-357 DOI:http://dx.doi.org/10.12944/CWE.4.2.13
Copy the following to cite this URL:
Malik M.K, Kumar P, Seth R, Rishi S. Genotoxic effect of paper mill effluent on chromosomes of fish Channa punctatus. Curr World Environ 2009;4(2):353-357. Available from: http://www.cwejournal.org/?p=981
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2009-09-30 |
|---|---|
| Accepted: | 2009-11-10 |
Introduction
Intensive industrialization has led to the introduction of a large number of chemicals in the biosphere, the habitat of all living beings, including man. The industrial units drain their effluents directly into the water bodies which pose a serious threat to the aquatic flora and fauna. The industrial effluents carry many toxic substances which are capable of affecting the genetic material of the organisms, leading to immediate and inherited mutation. A variety of somatic diseases like cell death, immunological impairment, ineffective tissue repair, premature ageing and carcinogenesis may also be the result of the chemicals in the effluents. Therefore, it is essential to assess the genotoxic potential of these effluents.
Effluents from the factories/industries are introduced into the aquatic environment directly or as run-off during rainfall. The water bodies thereby become sinks for chemical residues (Frank and Logan, 1988; Kushwaha et al., 2003; Steinhagen et al., 2004). The toxic nature of these chemicals manifests in a number of ways. Genotoxicity of pesticides and heavy metals in fishes have been demonstrated by number of workers (Das and Nanda, 1986; Sabti et al., 1994; Sabti and Metcalfe, 1995; Rishi and Grewal, 1995; Mathew and Jahageerdar, 1999; Clarice et al., 2001; Maples and Bain, 2004). However, till date little has been done to evaluate genotoxic effect of the paper mill effluent. The fresh water fish Channa punctatus has proved to be a very good model for genotoxic studies as it possesses a small number of chromosomes of relatively larger size (Rishi and Grewal, 1995). Therefore, in the present study fish Channa punctatus has been chosen as a test model to study the genotoxic effect of effluent emanating from a paper mill.
Materials and Methods
Specimens of fish Channa punctatus weighing 50- 60 gm. were collected from ponds near Kurukshetra University and were acclimatized to laboratory conditions (pH 7.0 - 7.2, temperature 27 - 29C, D.O. 5.97, C.O.D. 65.1,Hardness 73.6, Alkalinity 75 and T.D.S. 228.3) in large well aerated glass aquaria of 100 liter capacity for 15 days prior to exposure to test chemical. Fishes were fed on minced goat liver. Every effort was made to provide optimal condition for fish and no mortality occurred during conditioning period. The samples of effluent were collected from Sara swati Paper Mill; Kurukshetra (Haryana).The composition of collected effluent was as suspended solids 192. 0 mg/l, ammonical nitrogen 4.0 mg/l, dissolved solid 1462.0 mg/l, chlorides160.0 mg/l, sulphides (as SO4) 212.3 mg/l, sodium 29.77%, oil and grease in small amounts, COD 256.0 mg/l, BOD 73.0 mg/l and pH 8.67.
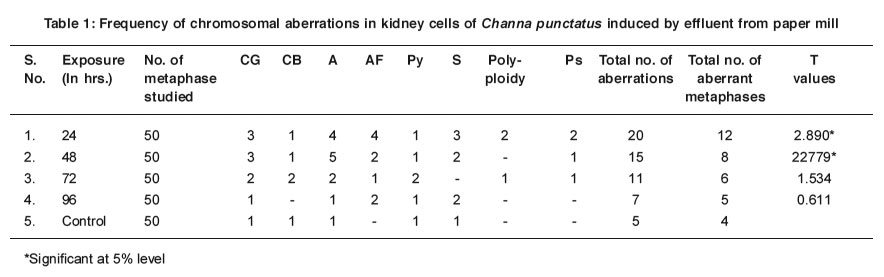 |
Table 1: Frequency of chromosomal aberrations in kidney cells of Channa punctatus induced by effluent from paper mill Click here to view table |
Five acclimatized specimens in a batch were placed in aquaria containing undiluted effluent. Mass mortality was observed within a few hours. Therefore, specimens in batches of 5 each were kept in aquaria containing effluent diluted with water (1:10) for 24, 48, 72 and 96 hours. For a negative control, one specimen for each duration was kept under similar conditions but in normal fresh water. For a positive control, a few specimens were injected with 0.025% Mitomycin C (MC) @ I ml/ 100 gm. body weight of fish.
 |
Figure 1 Somatic metaphase plate of Channa punctatus showing Acentric fragment (AF), Precocious separation (PS) and Pycnosis (Py). Click here to view figure |
For chromosomal preparation fish kidney was taken out and suspended in Fish BSS. Then 0.1 % colchicine was added @ 0.01 ml/10 BSS. The suspension was centrifuged and the pellet was suspended in 0.56 % KCL for 25-30 minutes. Finally slides were prepared following the usual hypotonic - acetic- alcohol, air -drying technique. The data were analyzed by using Student t- test at 5% level of significance.
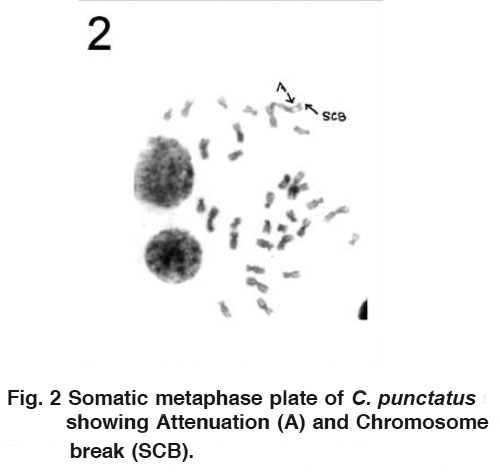 |
Figure 2 Somatic metaphase plate of C. punctatus showing Attenuation (A) and Chromosome break (SCB). Click here to view figure |
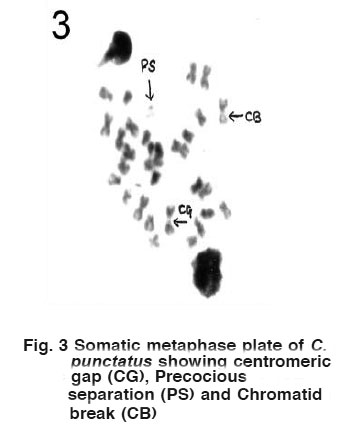 |
Figure 3 Somatic metaphase plate of C. punctatus showing centromeric gap (CG), Precocious separation (PS) and Chromatid break (CB) Click here to view figure |
Results
Various types of chromosomal aberrations viz. centromeric gap, chromatid break, attenuation, acentric fragment, pycnosis, stickiness, stubbed arm, precocious separation and polyploidy were obser ved in specimens of Channa punctatus treated with the paper mill effluent( Figs. 1,2,3,4&5). The total number of aberrations and the total number of metaphases spread with chromosomal aberrations were observed to be significantly (P<0.05) higher in the treated specimens than controls at 24h and 48 h exposure (Table 1). However, the differences were non - significant (P>0.05) at 72 h and 96 h exposure. Out of 50 metaphases scored from each treatment, there were a total of 20 aberrations after 24 h, 15 after 48 h, 11 after 72 hrs and 7 after 96 h of exposure. The results also indicated a regular decline in number of aberrations with the increase in duration of exposure to the effluent.
 |
Figure 4 Somatic metaphase plate of C. punctatus showing Stubbed arms (SA). Click here to view figure |
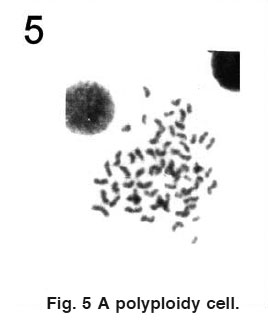 |
Figure 5 A polyploidy cell. Click here to view figure |
Discussions
Fish provide an excellent source of mater ial for the study of the mutagenic or carcinogenic potential of water samples as they are aquatic ve r tebrate, which can metabolize, concentrate and store water pollutants. There are many cytogenetic end points that can be used as an indication of exposure to genotoxic substances in aquatic organisms. Fish chromosomes can act as an important bio -indicator of the presence of pollutant in the water. Thus chromosomes studies in fishes have become increasingly important now a day, for evaluation of genotoxic potential of pollutants. Most of the genotoxic work has been carried out on fishes using various pesticides, weedicides and herbicides (Plowa et al., 1984; Pino et al., 1988; Tripathy and Patnaik, 1992; Rishi and Grewal, 1995; Ali and Ahmad, 1998). The present results on chromosome aberrations are in accordance with the observations made by earlier workers in Anabas (Biswas and Manna, 1989), Tilapia (Biswas and Manna, 1992), Channa punctatus (Rishi and Grewal, 1995; Porichha et al., 1996; Ali and Ahmad 1998; Tolga et al., 2005). So it has been opined that most of the chemicals and drugs produce similar aberrations which cannot be explained by a specific bio- chemical interaction between the genotoxic agent and the DNA of the cell of the treated specimen (Manna, 1990). Bhattachar ya and Patnaik (1996) studied the mutagenic effects of effluents from a paper mill on the micronuclei of black rat, Rattus rattus and observed that this effluent is highly genotoxic and probably sulphur is the major genotoxic element. Similarly, Yadav and Trivedi (2006) observed that genotoxic potential of chromium (VI) in Channa punctatus is increased initially and then decreased gradually. In the initial stage chromium may damage the cell and the genetic material may induce more chromosomal aberrations but later on with increase in exposure time animals developed a mechanism by which they accumulate chromium in their body and the cells in which the chromosomal aberrations formed, fail to divide and multiply.
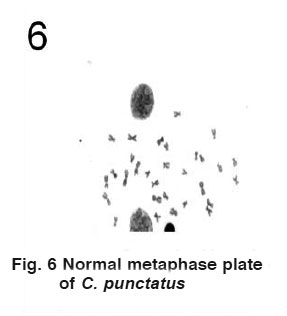 |
Figure 6 Normal metaphase plate of C. punctatus Click here to view figure |
The results of present study also confirm the genotoxic potential of the paper mill effluent on chromosome of fresh water fish Channa punctatus. A comparison of average values of chromosomal aberrations indicates that the treatment during four consecutive days exhibits a marked increase in these aberrations over the control values. The percentage of aberrations was highest in fish treated for 24 hrs, thereafter the values showed a decrease with increase in the period of treatment. This may be due to the fact that the effluent degrades easily. However, these values never fall below the ones observed for the control fish. This decrease in aberrations correlates with the fact that DNA undergoes repair as the contents of the effluent degrade. However, the effluent is being regularly added to the water bodies in the natural environment and the fish remains continuously in contact with higher concentration of the chemicals .Thus, there is a greater risk in nature than observed under the experimental conditions. Therefore, further studies are required to arrive at a definite conclusion and to find out the active principal of genotoxicity of the paper mill effluent.
Acknowledgements
The authors are thankful to the Chairman, Department of Zoology, Kurukshetra University Kurukshetra for providing necessary laboratory facilities.
References
- Frank, R. and Login, L. Pesticide and industrial chemical residues at the mouth of the Grand Saugeen and Thames Rivers, Ontario, Canada. Arch. Environ. Contam. Toxicol.(1988) 17: 741-754.
- Kushwaha, B., Nagpure, N.S., Srivastava, S., Ravindra, K. and Verma, M.S. Variation of micronuclei in peripheral blood cells of Channa punctatus. Indian J. Animal Sci.(2003) 73: 1192-93.
- Steinhagen, D., Helmus, T. and Maurer, S. Effect of Hexavalent carcinogenic chromium on carp Cyprinus carpio immune cells. Dis. Aquat. Organ,(2004) 23: 155-61.
- Das, R.K. and Nanda, N.K. Induction of micronuclei in peripheral erythrocytes of fish Heteropneustes fossilis by Mitomycin-C and paper mill effluent. Mutat. Res.(1986) 175: 67-71.
- Sabti, K., Franko, M., Andrijanie, B., Kenz, S. and Stegnar, P. Chromium induced micronuclei in fish. J. Appl Toxicol.(1994) 14: 333-6.
- Sabti, K. and Metcalfe, C.D. Fish micronuclei for assessing genotoxicity in water. Mutat. Res.(1995) 343: 121-135.
- Rishi, K.K. and Grewal, S. Chromosome aberration test for the insecticide, dichlorovs on fish chromosomes. Mutat. Res.(1995) 344: 124-127.
- Mathew, N.P. and Jahageerdar, S. Effect of heavy metal on the karyotype of Channa punctatus. Indian J. Fish,(1999) 46: 167-72.
- Clarice, T. L., Patricia, M.R., Nara, R.T. and Ber nardoo, E. Evaluation of basal micronucleus frequency and hexavalent chromium effects in fish er ythrocytes. Environ. Toxicol. Chem.(2001) 20: 1320-24.
- Maples, N.L. and Bain, L.J. Tr ivalent chromium alters gene expression in the Fundulus heteroclitus. Environ. Toxicol. Chem.(2004) 23: 626-31.
- Plowa, M.J., Wagner, E.Q., Gentile, G.J. and Gentile, J.M. An evaluation of the genotoxic properties of herbicides following plant and animal activation. Mutat. Res.(1984) 136: 233 -245.
- Pino, A., Maura, A. and Gr ilo, P. DNA Damage in stomach, kidney, liver and lung of rats treated with atrazine. Mutat. Res.(1988) 209:145-147.
- Trapathy, N.K.and Patnaik, K.K. Studies on the genotoxicity of monochrotophos in somatic and germ line cells of Drosophila. Mutat. Res.(1992) 278: 23-29.
- Ali, N.M. and Ahmad, W. Effect of pentachlorphenol on chromosomes of a catfish, Heteropneustes fossilis. Indian J. Exp .Biol.(1998) 36: 304-307.
- Biswas, S. and Manna, G.K. The mutagenic potentiality of the bacterium Pseudomonas aeruginosa tested on climbing perch Anabas testudineus" Perspectives in Cytology and Geneties (Eds. G. K. Manna and U. Sinha)(1989) Vol. 6: 573-598
- Biswas, S. and Manna, G. K. The "Hay- Bacillus", Bacillus substilis as genotoxic agent in treated freshwater Tilapia. Perspectives in Cytology and Genetics (Eds. G.K. Manna and S.C. Roy )(1992) Vol . 7: 945-952.
- Porichha, S.K., Sarangi, P.K. and Prasad, R. Genotoxic effect of Chloropyriphos in Channa punctatus. Abstract pp. 65 IX th AICCG, Chandigarh (1996).
- Tolga, C., Natasha, N.G. and Victor, V.A. Induction of micronuclei and binuclei in blood, gill and liver cells of fishes subchronically exposed to cadmium chloride and copper sulphate. Food Chem. Toxicol.(2005) 43: 569-74.
- Manna, G.K., Convener' s Address : Impacts of Environment on Animals and Aquaculture (Eds. G.K.Manna and B.B Jana),1990: XI- XV
- Bhattachar ya, M. and Patnaik, S.C. Mutagenic effect of discharged effluents from a paper mill at choudwar tested through micronucleus assay in wild rat. Abstract pp. IXth AICCG, Chandigarh (1996).
- Yadav, K.K. and Trivedi, S.P. Evaluation of Genotoxic Potential of Chromium (VI) in Channa punctatus fish in ter ms of chromosomal aberrations. Asian Pacific J. Cancer Prev.(2006) 7: 472-67.






