Bioremediation of phenol and naphthalene by Bacillus species and Brachybacterium species isolated from pharma soil sample
A.M. Velmurugan1 * and C. Arunachalam1
DOI: http://dx.doi.org/10.12944/CWE.4.2.06
In this present study, Soil samples were collected from the pharma industries namely Sai Meera pharmaceuticals, Chennai. Samples were screened and totally 10 colonies were isolated in the Mineral Agar medium containing 10 mg of phenol and naphthalene and named as P1 to P10. The screened colonies were enriched in liquid minimal media containing phenol and naphthalene for 5 days. The enriched colonies streaked on the mineral agar plates containing increasing amount of phenol and naphthalene for potential strain. The potential strains were selected and named as P7 and P10. The potential strains were studied for degradation ability using 4-amino antipyrene method and the degradation ability were calculated by colorimetric O.D values at 600nm. Optimization of pH and temperature was carried out for the maximum degradation of chemical by the potential strains. The P7 and P10 strains showed better growth in neutral pH. P7 strain showed good growth at 28°C and 37°C but P10 strain shown better growth at 28°C. Immobilization of the potential strains was carried out for the better and maximum degradation and their degradation ability was calculated by 4-amino antipyrene method. Immobilized cells can be reused for further degradation. The potential strains P7 was identified as Bacillus sp and P10 was identified as Brachybacterium sp.
Copy the following to cite this article:
Velmurugan A.M, Arunachalam C. Bioremediation of phenol and naphthalene by Bacillus species and Brachybacterium species isolated from pharma soil sample. Curr World Environ 2009;4(2):299-306 DOI:http://dx.doi.org/10.12944/CWE.4.2.06
Copy the following to cite this URL:
Velmurugan A.M, Arunachalam C. Bioremediation of phenol and naphthalene by Bacillus species and Brachybacterium species isolated from pharma soil sample. Curr World Environ 2009;4(2):299-306. Available from: http://www.cwejournal.org/?p=965
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2009-09-10 |
|---|---|
| Accepted: | 2009-11-09 |
Introduction
Chemicals are part of the modern life. They are part of all spheres of human life and used as pharmaceuticals, pesticides, detergents, fertilizers, dyes, preservatives, food additives etc. Pharmaceutical wastes produced on a daily basis may be land filled provided that they are dispersed in large quantities of general waste. Cytotoxic and narcotic drugs, however, should never be land filled, even in small quantities. Mild liquid or semi-liquid pharmaceuticals, such as solutions containing vitamins, cough syrups, intravenous solutions, eye drops, etc. (but not antibiotics or cytotoxic drugs), may be diluted in a large bow of water and discharged into municipal sewers. Petroleum hydrocarbons can be divided into four classes namely saturates, aromatics, the asphaltenes (phenol, fatty acids, ketones, esters and porphyrines), and the resins (pyridines, quinolines, carbazoles, sulfoxides and amides).
Phenol, C6H5OH or hydroxybenzene, is an aromatic molecule containing hydroxyl group attached to the benzene ring structure. Phenol commonly known as carbolic acid has a molecular weight of 94.11 gm/M 1. It has a melting point of 43°C and forms white to colorless crystals, colourless to pink solid or thick liquid. Phenol is a constituent of coal tar, and is formed during decomposition of organic materials. Ingestion of even small amounts may cause vomiting, circulatory problems, paralysis, convulsions, and coma. Phenol affects the nervous system and key organs, i.e. spleen, pancreas and kidneys.
Naphthalene PAHs including naphthalene (2 rings), fluorene (3 rings), benzo (a) anthracene, chrysene, fluoranthene, pyrene (4 rings), and benzo (a) pyrene (5 rings). Exposed to naphthalene vapours causes Nausea, vomiting, abdominal pain, and anemia Increased serum bilirubin, met hemoglobin, Heinz bodies, and fragmented red blood cells hematuria, anemia, restlessness, and liver enlargement2.
Microbial degradation In aerobic respiration, oxygen acts as the electron acceptor. Molecular oxygen is a reactant for oxygenase enzymes and is incorporated into the final products. In anaerobic respiration, different inorganic electron acceptors are released such as NO3, SO4, SO, CO2 and Fe3 during microbial degradation. Following microorganism is responsible for the degradation of phenol and naphthalene. Acinetobacter sp.,Arthrobacter sp.,3 Bacillus sp.4 Micrococcus sp., Nocardia sp. Pseudomonas sp.5 P. pictorum- NICM- 2077, Rhodococcus sp, DCB-p0610 Fusarium - F. FE11 Penicillimm AF2, AF4, 6 Coprinus sp. Candida tropicalis. Considering the versatile nature of microorganisms as a good source of degrading the wastes, especially the pharmaceutical residues an attempt has been made to understand the utility of microorganisms for the degradation of pharmaceutical waste.
Material and Methods
Collection of Sample
The soil sample was collected from Sai Meera pharma industry at Chennai. Sample was collected in polythene bag by using pre-sterilized spatula.
Screening for Phenol and Naphthalene Degrading Organism
One gm of soil sample was serially diluted up to 106 dilution, and spreaded on minimal agar medium containing 10 mg/ 100 ml phenol and naphthalene which was added after sterilization, and the plates were kept for incubation at 28°C for 5 days7.
Enrichment of the Isolated Strains
The 18 hrs selected cultures were prepared in nutrient broth. One ml of this turbid broth was taken and added into 49 ml of liquid mineral medium supplemented with 10 mg of phenol and naphthalene in a sterile 250 ml conical flask and kept in a rotary shaker at 120 rpm for 5 days8.
Selection of Potential Phenol and Naphthalene Degrading Strains (Plate Method)
Minimal agar medium was prepared with increasing concentration of selected phenol and naphthalene as follows 10 mg/100 ml, 20 mg/100 ml, 30 mg/100 ml, 40 mg/100 ml, and 50 mg/100 ml of enriched cultures were inoculated on minimal agar media. Plates were incubated at room temperature for 48-72 hrs.8
Degradation by Free Cells
The selected potential isolates were inoculated in 10 ml nutrient broth, after 18 hrs of incubation 5 ml of this nutrient broth was added to 95 ml of minimal media containing different concentrations of phenol and naphthalene individually30mg/100ml, 40mg/100 ml, 50mg / 100ml. 3 ml of sample were removed every one hour and microbial growth was measured using colorimeter at 600 nm. The aliquot samples were centrifuged at 1000 rpm for 5 min and supernatant was obtained for the study of degradation ability of phenol and naphthalene using 4-amino antipyrenemethod4.
4-Amino Antipyrene Method
The phenol and naphthalene compounds reacts with 4-amino antipyrene at pH 7.9 ± 0.1 in the presence of potassium ferriccyanide (K3Fe (CN) 6) and forms a pink colored antipyrene dye. This dye kept in aqueous solution and the absorbance was monitored at 500 nm.4
Optimization of Phenol and Naphthalene Degradation by Potential Strain Effect of pH and Temperature
The minimal agar medium was prepared with different pH (5, 7 and 9) and different temperature (28ºC, 37ºC, 45ºC) for 48-72 hrs also supplemented with 50mg phenol and naphthalene separately.
Preparation of Immobilized Cells
Phenol and naphthalene (0.01%) as a sole source of carbon and kept in rotary shaker at 120rpm for 72 hrs at 30ºC. 3gm of sodium alginate was dissolved in 100 ml of distilled water and mixed thoroughly and autoclaved. Then it was stirred with 3 ml of potential broth culture using magnetic stirrer. Then the final mixture was extruded through a needle into 200 ml of 0.05M CaCl2 solution to yield 3 to 4 mm diameter alginate beads. After 20 min, the beads were filtered out and stored in 0.05 % CaCl2 solution in the refrigerator.9
Degradation of Phenol and Naphthalene by Immobilized Cells
15 gm of immobilized beads were placed into 100 ml of mineral medium containing the desired amount of phenol (30 mg/100 ml, 40 mg/ 100 ml, and 50 mg/100 ml). Aliquot samples of the broth culture were taken at regular interval for the study of degradation by 4-amino antipyrene methods.
Identification of Potential Strains
Phenotypic characteristics such as micromorphology (gram staining, capsule staining endospore staining and motility), cultural characteristics (on basal media, differential media and selective media), and biochemical characteristics (catalase, oxidase, IMViC). Based on the results of studied phenotypic characteristics all the selected bacterial isolates are identified with the help of Bergey's Manual of Determinative Bacteriology10.
Results and Discussion
Screening for Phenol and Naphthalene Degrading Organism
During the third day of incubation, two colonies from phenol containing plate and three colonies from naphthalene containing plate were observed and the plates were further incubated. After 5 days, another two colonies from phenol containing plate and three colonies from naphthalene containing plate morphologically different colonies were observed. Totally 10 isolates were selected as phenol and naphthalene degrading bacterial colonies and named as strain P1 to P10. The stock cultures for the selected strains were maintained. Rigo and Alegre, used phenol as the sole carbon source in the defined mineral salt medium for the isolation process7, in the present study were used as phenol and naphthalene as the sole carbon source it is the screening method for the degradation.
Selection of Potential Phenol and Naphthalene Degrading Strains
The enrichment medium which is used for the enrichment of an isolated strains from mixed cultures which show good variations of species in mixed cultures8. In this study, Minimal medium was used forthe enrichment purpose. Better growth was observed after five days. After incubation, all the strains namely P1 to P10 shows growth in 10 mg of phenol and naphthalene, but P7 and P10 show maximum degradation against all the concentrations of phenol and naphthalene. From these, P7 and P10 were selected as potential strains and used further studies (Table 1). On serial dilution two colonies from phenol plates and three colonies from naphthalene plates were observed within two days, in the next three days two colonies from phenol and three colonies from naphthalene were isolated. Totally ten colonies were observed on the mineral salt medium.
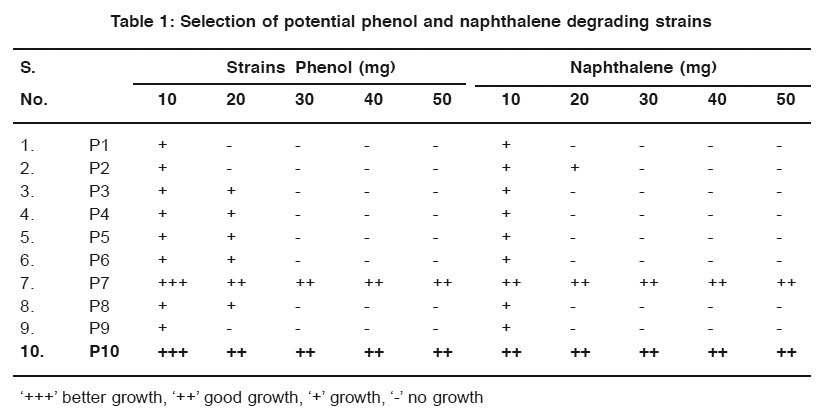 |
Table 1: Selection of potential phenol and naphthalene degrading strains Click here to view table |
Degradation by Free Cells (4-Amino Antipyrene Method)
The phenol and naphthalene in different concentration were added to mineral media for degradation by the potential isolates was studied by the colour reaction of chemical (phenol and naphthalene) with potassium ferric cyanide at pH 7.9. The optical density (O.D) value was measured for the change in colour by using colorimeter. For the degradation studies, various concentrations of phenol and toluene in (25 mg / L, 50 mg / L, 75 mg / L and 100 mg / L) were used. It is beneficial for the study of biodegradation to quantity their pollutant effect studied4. For this present study, increasing concentration of phenol and naphthalene such as 30 mg/ 100ml, 40 mg/ 100ml and 50 mg/ 100ml of phenol and naphthalene were used for the degradation process. Out of the two strains P7 and P10 were selected as potential strains. (Fig. 1 to 4).
Optimization of Phenol and Naphthalene Degradation by Potential Strain P7 and P10 Effect of pH
After incubation, the plates containing both phenol and naphthalene in different pH condition were observed and the better growth was observed by P7 and P10 at neutral pH in the phenol plate and neutral pH in naphthalene plate. (Table 2 & 3).
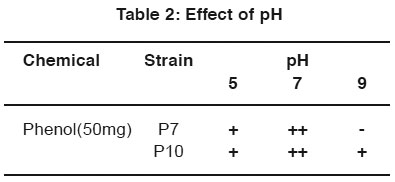 |
Table 2: Effect of pH Click here to view table |
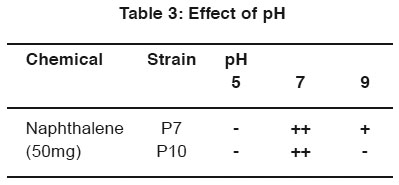 |
Table 3: Effect of pH Click here to view table |
Effect of Temperature
After incubation, the plates containing both phenol and naphthalene in different temperature conditions were observed. The strains P7 and P10 indicate better growth in 28°C and 37°C in phenol plate and 28°C in naphthalene plate. (Table 4 & 5).
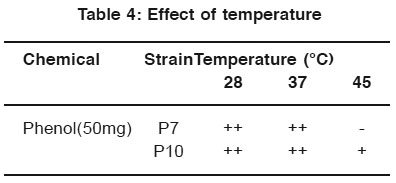 |
Table 4: Effect of temperature Click here to view table |
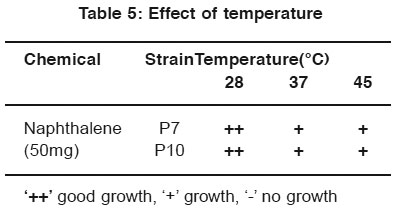 |
Table 5: Effect of temperature Click here to view table |
For the optimization process, different pH, temperature and enzymatic activity of Serratia plymuthica isolate from sludge sample reported11 which shows the optimization of the degradation at certain pH and temperature optimized the cells by applying different pH and temperature for the microbial degradation of phenol12. In the same way in the work degradation of phenol by the influence of optimum pH was already reported13. In this study P7 and P10 strain show better growth in neutral pH. P7 and P10 strain shown better growth in 28°C and 37°C for phenol degradation. P7 and P10 strain shown better growth in 28°C for naphthalene degradation.
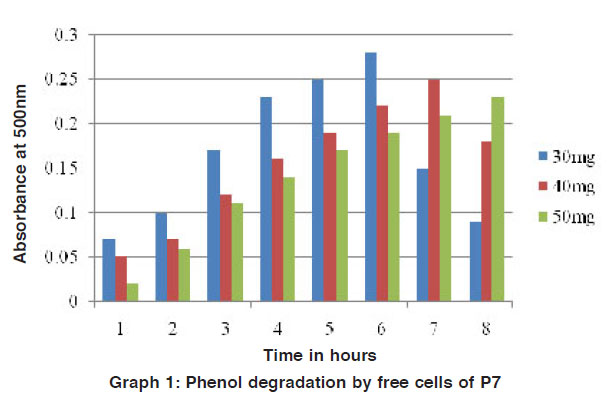 |
Graph 1: Phenol degradation by free cells of P7 Click here to view graph |
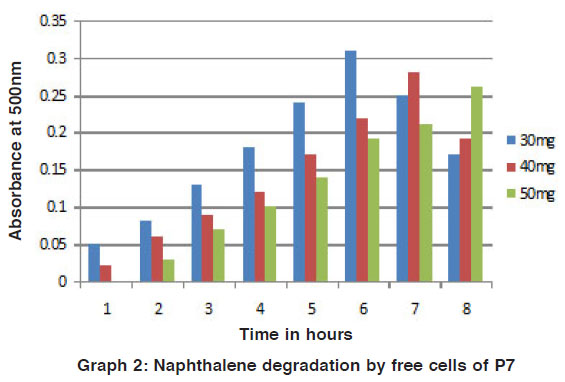 |
Graph 2: Naphthalene degradation by free cells of P7 Click here to view graph |
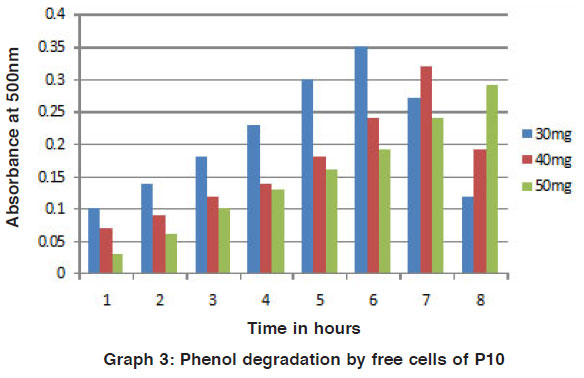 |
Graph 3: Phenol degradation by free cells of P10 Click here to view graph |
 |
Graph 4: Naphthalene degradation by free cells of P10 Click here to view graph |
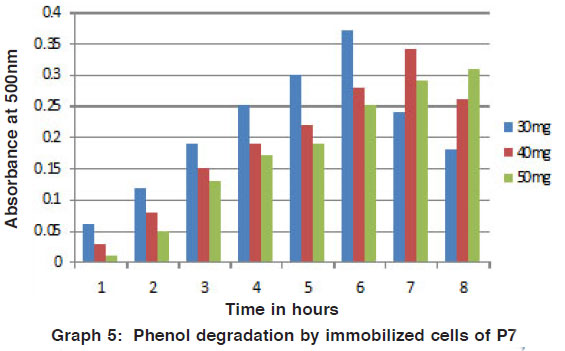 |
Graph 5: Phenol degradation by immobilized cells of P7 Click here to view graph |
 |
Graph 6: Naphthalene degradation by immobilized cells of P7 Click here to view graph |
Degradation studies by immobilized cells (4-amino antipyrene method)
The degradation of phenol and naphthalene in different concentration were added to mineral media by the potential isolates was studied by the colour reaction of chemical (phenol and naphthalene) with potassium ferric cyanide at pH 7.9. The optical density value was measured for the change in colour by using colorimeter. Recently, for the better advantage in degradation of various chemicals the free microbial cells were immobilized. So that they can be reused for the cycle and compare to free cells these shows on better results. (Figs. 5 to 8) Arthrobacter sp is immobilized for the phenol degradation studied14. Free and alginate entrapped Pseudomonas sp is used for the naphthalene biodegradation15.
Identification of Potential Strain
Based on the phenotypic studied, the potential strains were identified as Bacillus sp (P7). Brachybacterium sp (P10). In this study, Bacillus (P7) and Brachybacterium (P10) are the potential strain for degradation process. In which, Bacillus is already reported in the phenol and toluene degradation4 and Brachybacterium is a new strain to degrade phenol and naphthalene.
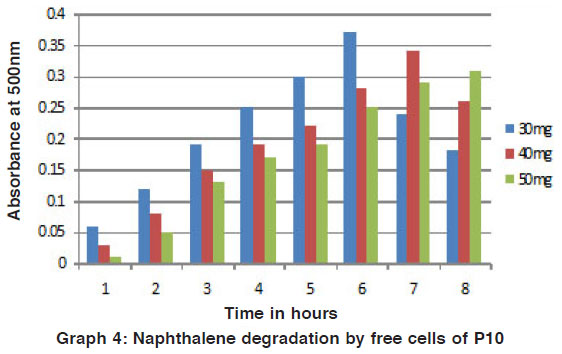 |
Graph 4: Naphthalene degradation by free cells of P10 Click here to view graph |
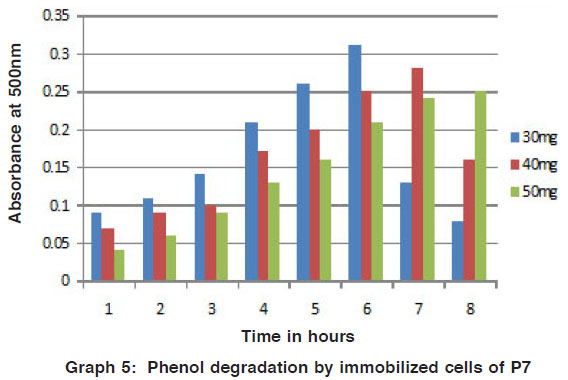 |
Graph 5: Phenol degradation by immobilized cells of P7 Click here to view graph |
Acknowledgments
I wish to sincerely record my deepest gratitude to Dr. K.R. Venkatesan M.A., M.Phil., Ph.D., Principal, Sri Sankara Arts and Science College, Kanchipuram for their valuable and enthusiastic encouragement at very state of this work. I express my sincere thank to Dr. R. Balagurunathan for his support during this research endeavor.
References
1. Serradilla, P., J. A., R. J. Lujan, and M. D. L. Castro. Anal Bioanal Chem. 392: (2008) 1241-1248..
2. Aronson, D., M. Citra, K. Shuler, H. Printup, and P. H. Howard. A summary of field and laboratory studies.(1999).
3. Kar, S., T. Swaminathan, and A. Baradarajan. Bioproc. Eng.(1996) 15(4): 195-199.
4. Prasanna. N., N. Saravanan, P. Geetha, M. Shanmugaaprakesh, and P. Rajasekaran. Ad. Biotech.(2008) 20 24.
5. Kang, M. H., and J. M. Park. Chem. Technol. Biotechnol.(1997) 69: 226-230.
6. Santos, V. L., and V. R. Linardi, Proc. Biochem. (2004) 39: 1001- 1006.
7. Rigo, M., and R. M. Alegre. Folia Microbial.(2004) 49(1): 41-45.
8. Yang and C. M. Lee. Biodegradation. (2008) 19: 329-336.
9. Parameswarappa, S., C. Karigar, and M. Nagenahalli. Biodegradation (2008) 19: 137-144.
10. John G. Holt, Noel. R. Krieg, Peter H. A. Sneath, James T. Staley and Stanley T. Williams.Determinative Bacteriology, 9th edition, Williams & Wilkins. (1994).
11. Pradhan, N., and A. O. Ingle. Int. Biodeterioration and Biodegradation.(2007) 60: 103-108.
12. Agarry, S. E., B. O. Solomon, and S. K. Layokun. Am. J .Biotech. (2008) 7: 2409-2416.
13. Graham, N., C. C. Jiang, X. Z. Li, J. Q. Jiang and Z. Ma.Chemosphere(2004) 56: 949-956.
14. Karigar, C., A. Mahesh, M. Nagenahalli, and D. J. yun. Biodegradation.(2006)17: 47-55.
15. Seoud, M. A., and R. Maachi. Z. Natur. For sci.(2003) 58: 726-31.






