Assessment of water quality at Kalatoly coast and a Shrimp hatchery, Coxs bazar
Sheikh Aftab Uddin1 * , Md. Abdul Kader2 , Abuhena Mustafa Kamal3 , Ayesha Akhtar4 and Md. Shahadat Hossain5
1
Institute of Marine Sciences and Fisheries,
University of Chittagong,
Chittagong,
Bangladesh
DOI: http://dx.doi.org/10.12944/CWE.4.2.12
The physico-chemical parameters of water were assessed between Kalatoly coast and a shrimp hatchery to understand the present status of water quality. This study was conducted from January 2008 to June 2008 at Coxs Bazar, Bangladesh. Water samples were collected twice monthly from the Kalatoly coast. Similarly, the ultra violet treated and larval rearing tank water were also collected from a shrimp hatchery situated at Kalatoly. Water temperature, salinity, dissolved oxygen and pH were more or less similar both in sea water and UV treated water but were highly variable in the water collected from larval rearing tank (LRT). The water NO3-N NO2-N, PO4, Fe and total dissolved solids were moderately variable among the sea water, UV treated water and LRT water. Study suggests that the existing hatchery activities are sustainable in the coast of Kalatoly which is greatly influenced by the water quality of adjacent coastal water.
Copy the following to cite this article:
Aftabuddin S, Kader M.A, Kamal A.M, Akhtar A, Hossain M.S. Assessment of water quality at Kalatoly coast and a Shrimp hatchery, Coxs bazar. Curr World Environ 2009;4(2):347-352 DOI:http://dx.doi.org/10.12944/CWE.4.2.12
Copy the following to cite this URL:
Aftabuddin S, Kader M.A, Kamal A.M, Akhtar A, Hossain M.S. Assessment of water quality at Kalatoly coast and a Shrimp hatchery, Coxs bazar. Curr World Environ 2009;4(2):347-352. Available from: http://www.cwejournal.org/?p=979
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2009-07-30 |
|---|---|
| Accepted: | 2009-09-09 |
Shrimp farming in Bangladesh play a key role in the national economy since the early 1990s. Shrimp farming is practiced in Bangladesh as monostock of the black tiger shrimp Penaeus monodon (Uddin and Kader, 2006). Before 1990s, shrimp farmers only stocked natural post larvae and harvested the marketable size of shrimps while the culture system was totally dependent on natural seed supply. At present, the entire culture of tiger shrimps almost depends on hatchery produced post larvae which left a ser ious environmental consequences through their waste water discharge into the aquatic coastal ecosystem. The fluctuation of water parameters such as temperature, pH, salinity, dissolved oxygen, alkalinity and hardness above the nor mal range may deteriorate the ambient water quality that leads to shrimp diseases and other problems. Besides, water quality maintenance is of the greatest importance in the growth and survival of larvae, post larvae and brood stock. The physico-chemical parameters including water temperature, salinity, dissolved oxygen, pH and the micronutrients like nitrate, phosphate and silica play a significant role in P. monodon hatchery operation and management (Das et al., 1996). If any one of the above parameters fluctuates abruptly, the whole production may collapse.
The role of physico-chemical parameters such as salinity and water temperature in relation to survival, growth and production of P. monodon fry was studied by many researchers elsewhere (Chen,1985; Chakrabortty et al.,1986 and Boyd,1989). Furthermore, Wickins (1976) and Law (1988) reported that mortality rate of P. monodon post larvae was high at pH <6.0 and the recommended value was 7.5 to 8.5. The effect of ammonia on post larvae of P. monodon was investigated by Wickins (1976), Chen et al. (1986) and Law (1988). Their studies revealed that nitrite and nitrate may effect the growth of P. monodon larvae.
Therefore, successful hatchery operation of P. monodon depends on good water supply from the adjacent water environment and good quality management i.e. keeping the essential water parameters within suitable ranges. The present study was undertaken to assess the present status of water quality parameters at Kalatoly coast and a shr imp hatcher y namely Shahajalal Shr imp Hatchery at Cox's Bazar which could be valuable to understand the water quality of the hatchery zone where these types of studies are practically poor.
Materials and Methods
The present study was carried out in Kalatoly, Cox's Bazar coastal area and inside a P. monodon hatchery Shahajalal Shrimp Hatchery (Fig. 1). The hatchery drawns saline water from the sea, which is filtered through sand filters and chlorinated and dechlorinated before distribution to the larval tanks. Raw sea water and water samples from two points in the hatchery such as UV treated water and water from tanks rearing larval stages (PL7-PL15) were collected from 30 cm below the surface using sterile bottles. Sampling was done from January 2008 to June 2008. Sampling was accomplished twice monthly, and a total of 12 sampling trips were done and 36 samples were collected out over 6 months of study.
Surface water temperature, salinity and pH were measured in situ using a mercur y thermometer, refractrometer and a digital pH meter respectively. Total dissolved solid was measured by TDS meter of HANNA instr uments products (Singapore, Model No. HI-98302). Alkalinity was measured by alkalinity test kit (HANNA instrument product, Singapore, Model No. HI-98737). The micronutrients such as NO -N, NO -N, PO, and Fe were deter mined following the method of Chattopad ay (1998). DO was deter mined by Winkler's method (Barnes, 1959).
 |
Figure 1. The investigated area (•) where the present study done. Click here to view figure |
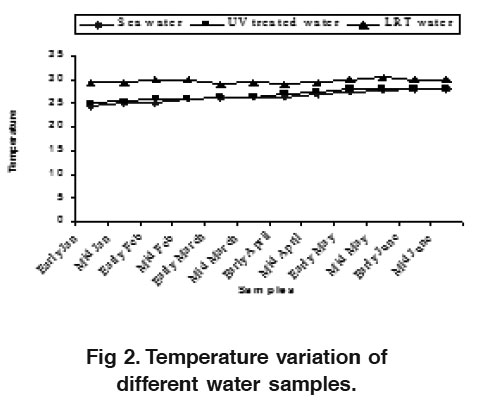 |
Figure 2. Temperature variation of different water samples. Click here to view figure |
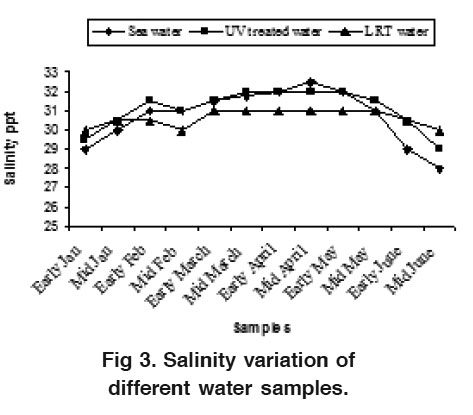 |
Figure 3. Salinity variation of different water samples. Click here to view figure |
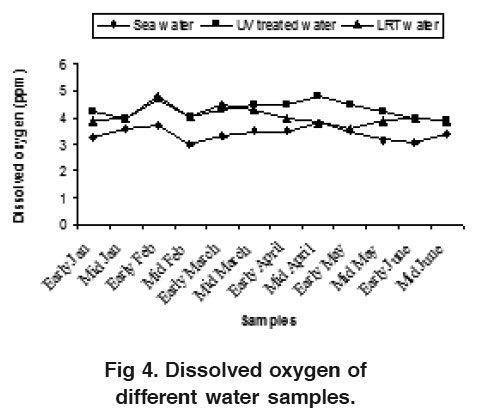 |
Figure 4. Dissolved oxygen of different water samples. Click here to view figure |
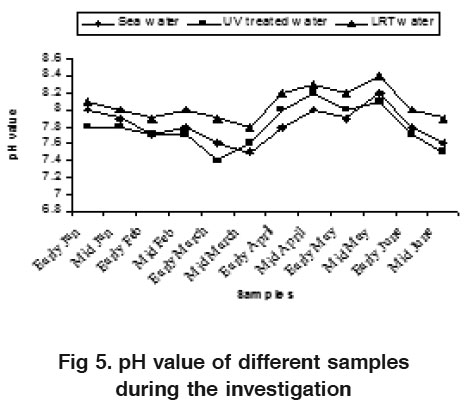 |
Figure 5. pH value of different samples during the investigation Click here to view figure |
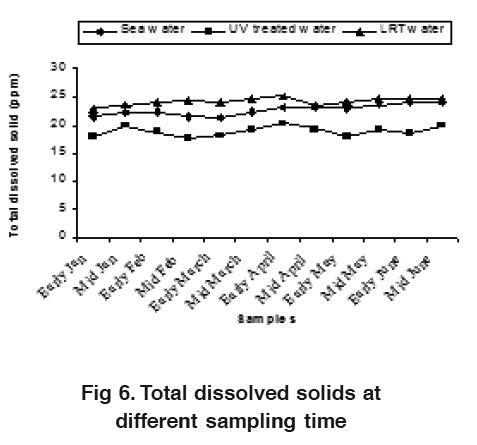 |
Figure 6. Total dissolved solids at different sampling time Click here to view figure |
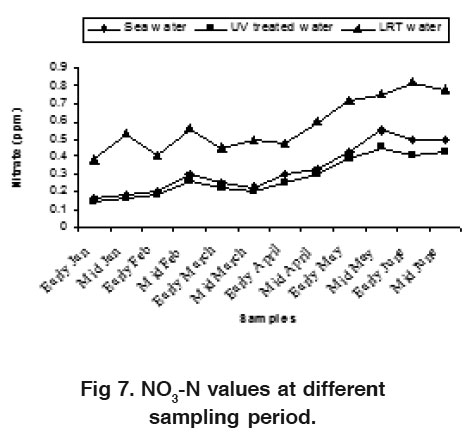 |
Figure 7. NO -N values at different sampling period. Click here to view figure |
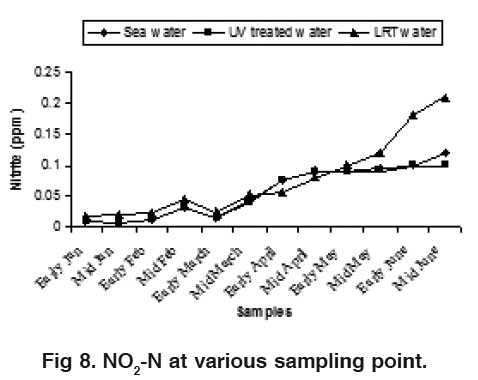 |
Figure 8. NO -N at various sampling point. Click here to view figure |
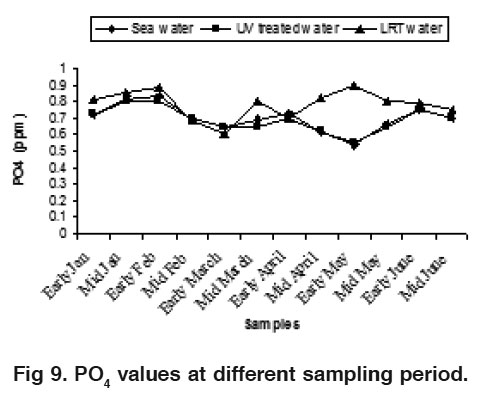 |
Figure 9. PO values at different sampling period. Click here to view figure |
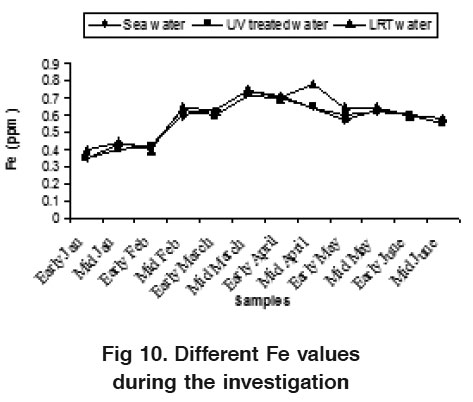 |
Figure 10. Different Fe values during the investigation Click here to view figure |
Results and Discussions
Water is the most vital component among the natural resources, and is crucial for the survival of all living organisms. Managing of water quality during the larval rearing phase is very important due to the sensitivity of larvae to the fluctuation of water parameters. The lowest temperature was observed in January (24.5°C) and the highest (28°C) in May for sea water (Fig. 2). There were major differences between the sea water and UV treated water (25°C-28.5°C) but the variation was not so remarkable in the water of LRT (29°C-30.5°C). This occurrence is probably due to indoor larval management practice of the P. monodon. The water salinity varied from 28.0 ppt to 32.5 ppt, 29.0 ppt to 32.0 ppt and 30.0 ppt to 31.0 ppt in sea water, UV treated water and LRT water respectively. The higher water salinity (32.5 ppt) was observed in April and lower (28 ppt) in middle of June for both sea water and UV treated water (Fig. 3). Moulting and consequent gr owth of postlar vae are highly stimulated by high salinity (Chen et al., 1989 and Ferraris et al., 1986). However, salinity was almost uniform for the water of LRT (30-31 ppt) due to larval rearing practices of P. monodon in hatchery. The present water temperature and salinity were found nearer to the range of 29°C to 30.5°C and 29 to 30 ppt for LRT water which coincided with the finding of Deb (1990) and Chang and Liao (1986).
Dissolved oxygen ranges from 3.10 to 3.72 ppm, 3.90 to 4.72 ppm and 3.82 to 4.80 ppm for sea water, UV treated water and LRT water respectively and fluctuated through out the study period (Fig. 4). The variation of DO could probably due to the effects of temperature, salinity, atmospheric pressure and other activities. Usually, DO is used by plants and animals for their respiration whereas, photosynthesis by phytoplankton and other aquatic plants is the primary source of DO in water. Chen et al. (1986) recommended DO level to be between 4.0-8.0 ppm for P. monodon post larvae rearing. The water pH were almost constant and varied from 7.5 to 8.2, 7.4 to 8.2 and 7.8 to 8.4 in sea water, UV treated water and LRT water respectively (Fig. 5). There were no remarkable variation for pH in sea water, UV treated water and LRT water. Generally, the variation in pH occurs due to the use of different artificial feed in larval rearing tank. The pH value also depends on the amount of carbon-di-oxide present in water and the addition of fresh water into the LRT. Chen et al. (1986) and Wickins (1976) reported that pH level between 7.17 and 8.34 was suitable for P. monodon post larvae and this coincides with the present findings.
Total dissolved solids (TDS) varied from 21.28 to 24.20 ppt, 17.65 to 20.25 ppt and 22.87 to 25.12 ppt in sea water, UV treated water and LRT water respectively (Fig. 6). Present study showed that the TDS values were higher in sea water and LRT water compared to the UV treated water. This variation could probably due to the existence of naturally suspended mater ials and feeding movement both in sea water and LRT. The total dissolved solid (TDS) was similar to the findings of Chen and Liu (1988).
The water NO -N varied from 0.17 to 0.55 ppm, 0.15 to 0.45 ppm and 0.38 to 0.82 ppm for sea water, UV treated water and LRT water during the study period, respectively (Fig. 7).These findings are slightly higher than the result of Das et al., (1996). The nitrate value in LRT water was higher than the sea water and UV treated water. The nitrification processes and the release of hydrogen ion during the hatchery rearing activities probably influence this phenomenon. Water NO3 -N varied from 0.006 to 0.12 ppm, 0.005 to 0.100 ppm and 0.018 to 0.21 ppm in sea water, UV treated water and LRT water respectively (Fig. 8). There was no distinct variation in sea water and UV treated water but the variation was clear in LRT water probably due to nitrification and other microbial activities occurring in the LRT water. Jayasankar and Muthu (1983) reported that an acute level of NO -N for P. indicus was 0.18 mg/l. Wickins (1976) reported that when nitrite nitrogen increased in the water of rearing tank, survival rate of P. monodon larvae gradually decreased. The highest safe level of NO2 -N recommended for P. monodon growth is 1.28 mg/l (Law, 1988) but the present findings are much lower than this. The concentration of PO4 was more or less similar in both sea water and UV treated water but slightly differed on LRT water during the month of May (Fig. 9). The present phosphate level was recorded 0.55 to 0.90 ppm in LRT water which is similar to the findings of Chen et al. (1986) and Das et al. (1996). The concentration of phosphate increases due to excretion of metabolites and heavy load of excess food, including both live phytoplankton and artificial diets. The concentration of Fe was more or less similar in both sea water and UV treated water but slightly differ on LRT water during the study period (Fig. 10). However, the introduction of fresh water may influence the concentration of Fe in LRT water at P. monodon hatchery.
Acknoledgements
The financial support for this study was given by "Research Cell", University of Chittagong, grant no. 4929/2007. Laboratory suppor t was provided by Institute of Marine Sciences and Fisheries.
References
- Bar nes, H., Apparatus and methods of Oceanography, part - 1 (Chemical) Academic press, London.(1959) 341.
- Boyd, C.E., Water quality management and aeration in shrimp farming. Fisheries and Allied Aquacultures, departmental Series No. 2, Alabama Agricultural Experiment Station, Auburn University, Alabama (1989).
- Cattopadhyay G.G., Chemical analysis of Fish pond soil and water, Daya publashing house, Delhi,(1998) 125.
- Chakaraborty R.K., Bhowmik, M. L., and Halder, D. D., Effect of change in salinity on the survival of Penaeus monodon postlarvae. Indian J. Fish,(1986) 33, 484-487.
- Chang J. H. and I. C. Liao., The effect of salinity on osmotic and ionic concentrations in the haemolymph of Penaeus monodon and Penaeus penicillatus. In: Proc: 1st Asian Fish. Forum. Asian Fish. Soc., Manila, Philippines,(1986) 633-636.
- Chen, H.C., Water quality criteria for farming the grass shrimp, Penaeus monodon. proc. 1st Intl. Conf. on Culture of Penaeid Shrimps. SFAFDEC, Aquaculture Dept. lloilo, Philippines(1985).
- Chen, J.C. and Liu, P.C., Feeding and nitrogen loading in an intensive prawn culture pond. Paper presented in Prawn Feed and Nutrient Symposium, National Taiwan Ocean University., Keelung, Taiwan, Dec.(1988) 11-12.
- Chen, J.C. Shin, T.S. and Lee, C.K., Effects of ammonia and nitrate on lar va l development of the shr imp Penaeus monodon. In: Proc. Ist Asain Fish. Forum. Asian Fish. Soc., Manila, Philippines,(1986) 657-662.
- Chen, J.C. Tu, C.C and Yang, W.S., Acute toxicity of ammonia to lar val Penaeus japonicus. J. Fish. Soc. Taiwan,(1989) 16(4), 261-270.
- Das, N.G., Sarwar, S.M.M. and Parvez, M.M., Obser vation on the water quality management of larval and post larval rearing tanks of P. monodon hatchery at Cox's Bazar; Bangladesh. Indian Journal of Fisheries,(1996) 43(3), 255-265.
- Deb, A.K., Artificial propagation of Penaeus monodon Fabricius, 1798 under recirculatory System in the prototype hatchery at Cox's Bazar. M. Sc. Thesis. Institute of Marine Sciences, University of Chittagong, Bangladesh.(1990) 105.
- Ferraris, R. P., Parado-Estepa, F.D. de Jesus, E. G. and Ladia, J.M., Osmoregulation in Penaeus monodon; effects of moulting and external salinity. In: Proc. 1st Asian Fish. Forum, Asian Fish. Soc., Manila, Philippines.,(1986) 637-640
- Law, A.T., Water quality requirements for Penaeus monodon culture. In. Proc. Sem. Mar. Prawn Farming in Malaysia. Serdang, Malaysia, Malaysian Fish. Society, 5th March, (1988).
- Uddin, S.A. and Kader M.A., The use of antibiotics in shr imp hatcher ies in Bangladesh. Journal of fisheries and Aquatic Science,(2006) l (1), 64-67.
- Wickins J.F.,The tolerance of warm water prawns to recirculated water. Aquaculture,(1976) 9, 19-37.






