The effect of gypsiferous water on biological activities of atrazine, 2, 4-D and metolachlor
C.F. Reinhardt1 * , S.L. Masike2 and L. Kanyomeka3
DOI: http://dx.doi.org/10.12944/CWE.4.1.02
Mine water effluents are a danger to the environment as they contain toxic materials such as heavy metals. In some cases though, efforts are made to neutralise the effluents so that the mine water is used for crop irrigation. However, suspected herbicide injury to crops has been reported in areas where gypsiferous water is used for irrigation, suggesting interaction between herbicides and gypsiferous water. Utilization of gypsiferous water for irrigation of crops is one major method promising to reduce the problem of effluent mine drainage disposal and also the shortage of irrigation water. Gypsiferous water is already used for irrigation of crops such as maize and wheat. The influence of gypsiferous water on key behavioural aspects of three important herbicides: atrazine, 2,4-D and metolachlor were assessed. Bioassay experiments were done to assess the bioactivity of the three herbicides in the presence or absence of gypsum in soil. The results showed that the activity of atrazine and 2,4-D was significantly increased in the presence of gypsum, while that of metolachlor was significantly reduced. These differential effects on herbicide activity would have important practical consequences for herbicide performance. Thus weed control efficacy, selectivity, and behaviour in the environment could be negatively affected
Copy the following to cite this article:
Reinhardt C.F, Masike S.L, Kanyomeka L. The effect of gypsiferous water on biological activities of atrazine, 2, 4-D and metolachlor. Curr World Environ 2009;4(1):9-13 DOI:http://dx.doi.org/10.12944/CWE.4.1.02
Copy the following to cite this URL:
Reinhardt C.F, Masike S.L, Kanyomeka L. The effect of gypsiferous water on biological activities of atrazine, 2, 4-D and metolachlor. Curr World Environ 2009;4(1):9-13. Available from: http://www.cwejournal.org/?p=877
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2009-01-06 |
|---|---|
| Accepted: | 2009-03-05 |
Introduction
Mine effluents disposal is a key threat to the environment in most mining areas of the world. In most cases the effluents are merely drained into some selected areas, and in worst cases it is drained into rivers or streams. Both ways the effluents cause negative effects on the environments, mainly because these effluents add heavy metals to the areas of effluents disposal. However, in some parts of the world efforts are being made to minimize this threat. Effluents are now used for agricultural pur poses. Effluents are treated with gypsum resulting in gypseferous water that can be safely used for crop irrigation. Gypsiferous water, i.e., water containing high levels of calcium sulphate, is used to irrigate crops such as maize and wheat on commercial scale in some parts of South Africa due to shortages of irrigation water (Jovanovic et al.,1998). However, gypserforous water may have negative interaction with certain herbicides used to control weeds. Gypsiferous water is saline due to the presence of Ca 2+, SO4 -2 and sometimes Mg 2+. These salts may have a detrimental effect on herbicide performance. Suspected herbicide injury to crops has been reported in areas irrigated with gypsiferous water, suggesting interaction between herbicides and gypsiferous water (Annandale et al.,1999).
Herbicides are widely used to control weeds in crop production systems, particularly in commercial farming. Chemical weed control represents the most practical way of coping with unwanted vegetation in this type of farming, and arguably at all levels of crop production (Ashton & Crafts, 1981; Klingman et al., 1982; Akobundu,1987) Atrazine (s-triazine), 2,4-D (phenoxy acetic acid) and metolachlor (chloroacetanilide) are popular herbicides registered for use in maize and wheat, and therefore they were included in this experiment. The behaviour of herbicides in soil and in plants is influenced by climatic and soil factors, type of herbicide, and plant species. The combined effect of these factors will determine the fate of a herbicide in the environment (Akobundu, 1987; Walk er, 1994; Conr ad et al ., 1999). The concentration of the herbicide at a vital location in the plant at any given time may determine herbicidal activity. If the amount of herbicide would be absorbed over an extended length of time it may have little or no effect, therefore the relative rates of herbicide uptake and subsequent metabolism in the plant system are important factors governing the sensitivity of both crops and weeds (Conrad et al., 1999).
Of particular importance is how herbicide- soil interactions could influence the following important criteria for herbicide performance: weed control efficacy, the tolerance of crop species, and the potential for herbicides to leach to groundwater. Herbicide interaction with gypsiferous water is a concern due to the presence of high concentrations of electrolytes that might react with herbicides in this type of water. The quantity and quality of water used as a carrier in herbicide application is known to influence the efficacy of certain herbicides (De Villiers, 2002).
Currently, there is information about the influence of gypsiferous water on the behaviour of herbicides. Should such interactions exist, findings of this present study will be useful in making recommendations for herbicide use in situations where gypsiferous water is used to irrigate crops. The objective of his study was to assess the effects of possible interaction between atrazine, 2,4-D or metolachlor and gypsiferous water on the availability of the herbicides for uptake by plants.
Material and Methods
For the purpose of this study, maize and wheat were considered priority crops since they are already commercially produced using irrigation with gypsiferous mine-water. The biological activity of atrazine, 2,4-D and metolachlor were assessed in separate pot exper iments conducted in a greenhouse at the Hatfield experimental farm of the University of Pretoria. Dose-response curves for the parameter dry matter yields were obtained with a herbicide concentration range of ten rates. Test species for assessing herbicide bioactivity were: wheat for atrazine, sorghum for metolachlor and sunflower for 2,4-D. Selection of a par ticular herbicide/test species combination was based on high sensitivity of the species to the respective herbicides. The rates for atrazine ranged from 0 - 0.225 mg kg-1 in a preliminary experiment, and in the subsequent experiment rates were adjusted to a range of 0 - 1.00 mg kg-1. Metolachlor rates ranged from 0 - 0.250 mg kg-1 and 0 - 275 mg kg-1, for the preliminary and the final experiment, respectively. Adjustments in concentration range were made because in the preliminary experiments not enough activity was found with the initial rates used for metolachlor, while the initial atrazine rates used were too high, and hence, elicited excessive bioactivity. The rates for 2,4-D were, however, not adjusted between experiments because they did not elicit too low or too high herbicide activity across major portions of the concentration range.
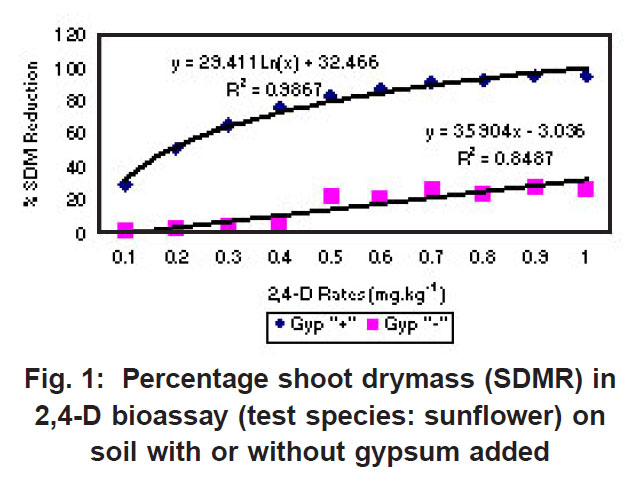 |
Figure 1: Percentage shoot drymass (SDMR) in 2,4-D bioassay (test species: sunflower) on soil with or without gypsum added Click here to view figure |
The sandy loam soil (Avalon) that was used in all the experiments consisted of 68.5 % sand, 8.2 % silt and 22.1 % clay, with a 0.4 % organic carbon content and pH 6.1. The two levels of the soil factor investigated were gypsum added to the soil, and no gypsum added (control). The or iginal soil was collected from the Hatfield experimental farm, and therefore, differences in soil properties were due only to the addition of gypsum. Five seeds were planted at a depth of 2 cm in 1 kg of soil contained in plastic pots. Plastic bags were used in each pot to prevent herbicides from leaching out.
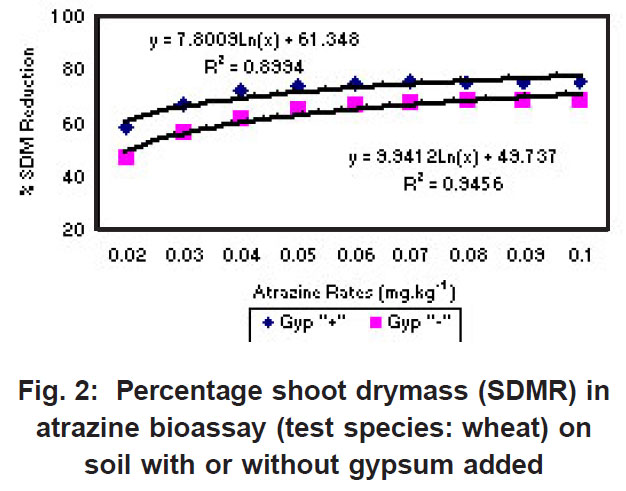 |
Figure 2: Percentage shoot drymass (SDMR) in atrazine bioassay (test species: wheat) on soil with or without gypsum added Click here to view figure |
Gypsum was thoroughly mixed with soil that had been air-dried and sieved through a 4-mm screen, at the rate of 10 % gypsum on a mass/ mass basis. The control treatment consisted of unamended soil. Pots were filled with either 1kg soil/gypsum mixture or 1 kg unamended soil. A specific volume of herbicide stock solution was thoroughly mixed with the 1kg soil by means of a mechanical mixer. Thereafter water was added to bring soil water content to 15%, which was the field capacity for this soil.
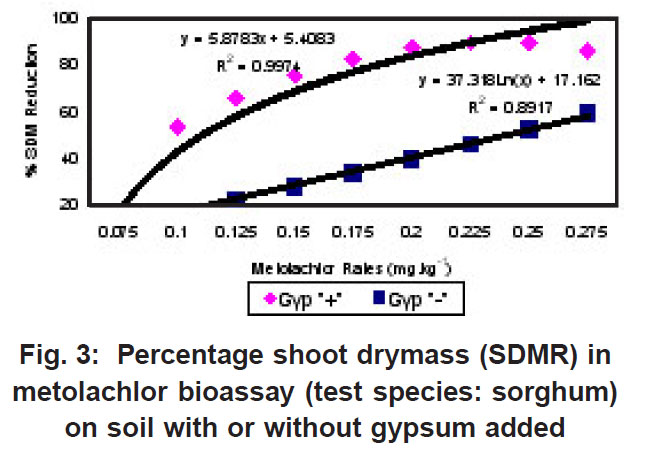 |
Figure 3: Percentage shoot drymass (SDMR) in metolachlor bioassay (test species: sorghum) on soil with or without gypsum added Click here to view figure |
Pots were weighed every two days to replenish soil water. After emergence, plants were thinned to three per pot to reduce intra-species competition. The experimental design in the greenhouse was completely randomised. Day and night temperatures varied between 25-30 oC and 15-18 oC, respectively. All pots were watered with nutrient solution (Nitsch, 1972) twice a week at 100 ml of nutrient solution per watering. Growth responses of the test species were expressed as percentage reduction in growth from the controls (zero herbicide). Plants were harvested four weeks after planting and shoot dr y mass measured. Analysis of variance (ANOVA) was done using the SAS programme. All data were subjected to analysis of variance, and means compared at P=0.05 using the Least Significant Difference test of Tukey. In striving for the best fit of dose-response curves, the highest R2-value and the lowest standard error, as well as the simplest functions were chosen, keeping in mind the biological meaning of the trend.
Results and Discussion
The percentage shoot dry mass reduction (SDMR) for the interaction soil x herbicide rate was significant for 2,4-D only, with reduction in dry mass accumulation ranging from 0-29 % for soil without gypsum, and 26-98 % for soil with gypsum. The interaction effect soil x herbicide rate for shoot dry mass (SDM) was significant in the case of all three herbicides, with mean SDM in the presence of 2,4- D ranging from 1.3-1.8 mg kg-1 for soil without gypsum, and 0-1.6 mg kg-1 in soil with gypsum added. For atrazine the mean SDM range was 0.05-0.18 mg kg-1 and 0.05-0.23 mg kg-1 for soil with gypsum and without it, respectively, while means for metolachlor ranged from 6-23 mg/kg and 2-27 mg/kg for both soil without gypsum and soil with gypsum. The 2,4-D exper iment indicated significantly greater biological activity in soil with gypsum added compared to where gypsum was not present (Fig. 1). Similarly, the biological activity of atrazine was significantly increased in the presence of gypsum (Fig. 2), and in the case of metolachor (Fig. 3), the effect was even more pronounced. The extent of reduction in growth caused by atrazine increased with increasing herbicide rates (Fig. 2).
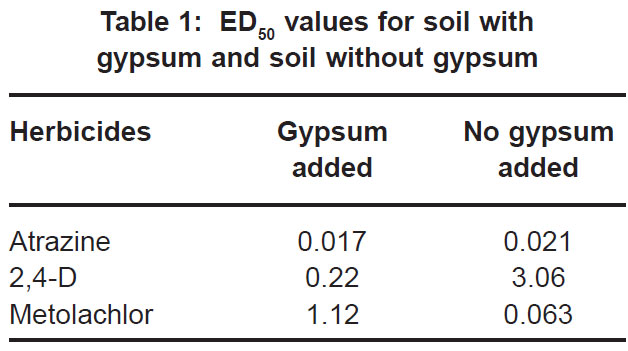 |
Table 1: ED50 values for soil with gypsum and soil without gypsum Click here to view table |
Anderson et al. (1980), Ehlers et al. (1987 & 1988), and Reinhardt et al. (1990) reported a strong negative correlation between the organic matter content and, to a lesser degree, the clay content of soils and atrazine bioactivity. Reinhardt (1993) reported that prediction of potential damage to susceptible crops should reflect the differential availability of atrazine in different soil types. Also, the amount of residues available for uptake by plants, and not merely total residue concentration in a particular soil should determine crop choice. Recropping intervals that are specified on labels of atrazine products neither reflect the variability in atrazine threshold values for sensitive crop species, nor the differential threshold concentrations for different soils (Reinhardt, 1993).
In contrast to the effect on the bioactivity of both atrazine and 2,4-D, the activity of metolachor was reduced in the presence of gypsum (Fig. 3). The magnitude of activity change was far greater in the case of metolachlor than for both 2,4-D (Fig. 1) and atrazine (Fig. 2). In the case of atrazine herbicide, the activity differences observed were so slight that it probably does not hold any practical consequences in ter ms of herbicide efficacy, selectivity or persistence. In contrast, the performance of metolachlor was dramatically affected, suggesting impor tant practical consequences. Significant reduction of metolachlor activity in the presence of gypsum implies that weed control by this herbicide will be poor on soils irrigated with water containing high levels of gypsum (calcium sulphate). Consequently, the finding does not suggest that selectivity of metolachlor towards the crop would be a concern. The effect on herbicide selectivity is also not an issue in the case of atrazine, because of the inherently high tolerance of the main crop in which it is applied, namely maize. Using best-fit regression equations the ED50 values (estimated dose causing 50% growth reduction) for each of atrazine, 2,4-D and metolachlor in soil plus gypsum, or in soil minus gypsum, were calculated as presented in Table 1 below.
For both atrazine and 2,4-D there was significantly greater biological activity in soil containing gypsum compared to where gypsum was absent. Increased bioactivity in the presence of gypsum suggests that the herbicides (atrazine and 2,4-D) were less strongly adsorbed to soil colloids in soil containing gypsum compared to soil without gypsum. Although this response will promote weed control efficacy, concomitant increased leaching potential poses a risk in terms of contamination of groundwater. In contrast, the activity of metolachlor was reduced in the presence of gypsum, and therefore, weed control efficiency was reduced.
Acknowledgements
Funds used for this study were made possible by the Water Research Commission (WRC) through Prof. J.G. Annandale’s project K5/1149. This support is gratefully acknowledged.
References
- Jovanovic, N.Z., Barnard, R.O., Rethman, N.F.G., & Annandale, J.G., Crops can be irrigated with lime-treated acid mine drainage.Water SA.(1998) 24(2): 113-122.
- Annandale, J.G., Jovanovic, N.Z., Benade, N., & Tanner, P.D., Modelling the long-term effect of irrigation with gypsiferous water on soil and water resources. Agr iculture, Ecosystems & Environment. (1999) 76: 109-119.
- Ashton, F.M., & Crafts, A.S., Mode of action of herbicides. John Wiley & Sons, New York (1981).
- Klingman, G.C., Ashton, F.M., & Noordhoff, L.J., Weed Science: Principle and Practices. John Wiley & Sons, New York (1982).
- Akobundu, I.O., Weed Science in the tropics: Principles and Practices.John Wiley & Sons, New York (1987).
- Walker, A., Herbicide behaviour in soils in Mediterranean climates. 5 th EWRS Mediterranean Symposium. “Weed control in sustainable agr iculture in the Mediterranean area”. Perugia (1994).
- Conrad, J.E., Colvin, C., Solilo, O., Gorgens, A., Weaver, J., & Reinhardt C. F. , Assessment of the impact of agricultural practices on the quality of groundwater resources in South Africa. Water Research Commission Report No. 641/1/99, Pretoria, South Africa (1999).
- De Villiers, B.L., The influence of salts in carrier water and adjuvants on glyphosate activity. PhD Thesis. University of Pretoria, South Africa (2002).
- Nitsch, J.P., Phytotrons: Past achievements and future needs. In: A.R., Rees, K.E., Cockshull, D.W., Hand, & R.G., Hurd (eds). Crop Processes in controlled environments. Academic Press, London (1972).
- Anderson, J.R., Stephenson, G.R. & Corke, C.T., Atrazine and cyanazine activity in Ontario and Manitoba soils. Can. J. Soil Sci.(1980) 60: 773-781.
- Ehlers, J.G., Reinhardt, C.F., & Nel, P.C., Atrazine activity under field conditions. Applied Plant Science.(1987) 1: 57-60.
- Ehlers, J.G., Reinhardt, C.F., & Nel, P.C., Effect of certain soil factors on the activity of atrazine. S. Afr. J. Plant Soil.(1988) 5: 32-36.
- REINHARDT, C.F., EHLERS, J.G., & NEL, P.C., Persistence of atrazine as affected by several soil properties. S. Afr. J. Plant Soil.(1990) 7: 182-187.
- Reinhardt, C.F., Biological activity and persistence of atrazine. PhD Thesis. University of Pretoria, Pretoria. South Africa (1993).






