Studies on fecundity of snow trout Schizothorax richardsonii (Gray) from the lotic bodies of Rajouri district (J&K).
Roopma Gandotra1 * , Ravi Shanker1 and Dalvinder Singh1
1
Department of Zoology,
University of Jammu,
Jammu and Kashmir
India
DOI: http://dx.doi.org/10.12944/CWE.4.1.19
The present work embodies studies on the reproductive capacity of Snow trout Schizothorax richardsonii collected from various streams of Rajouri district (J&K). About 50 fishes, ranging from 21.0 to 32.9 cm of total length, were put to the study of fecundity in relation to Fish length, Fish weight, Ovary length, Ovary weight and Ova diameter. The other aspects studied include relationship between Fish length and Ovary length, Ovary weight and Ova diameter. All the parameters were put to regression analysis. The higher values of correlation coefficient (re”0.9) in all the studied parameters indicated a strong correlation between them. However, the relationship between Fecundity and Fish length came out to be the highest indicating the dependence of Fecundity more on the length of fish body than other parameters.
Copy the following to cite this article:
Gandotra R, Shanker R, Singh D. Studies on fecundity of snow trout Schizothorax richardsonii (Gray) from the lotic bodies of Rajouri district (J&K). Curr World Environ 2009;4(1):127-132 DOI:http://dx.doi.org/10.12944/CWE.4.1.19
Copy the following to cite this URL:
Gandotra R, Shanker R, Singh D. Studies on fecundity of snow trout Schizothorax richardsonii (Gray) from the lotic bodies of Rajouri district (J&K). Curr World Environ 2009;4(1):127-132. Available from: http://www.cwejournal.org/?p=911
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2008-05-12 |
|---|---|
| Accepted: | 2008-08-17 |
Introduction
Fecundity of a fish can be defined as the number of eggs that are likely to be laid during one spawning period or the capacity of the fish in terms of egg production (Bagenal, 1968). It differs in different races of the same species. The measure of fecundity in fishes is a basic determinant of productivity and hence, contributes to fish culture and population dynamics. Important contributions to the study of fecundity in relation to the other parameters of body are made by Das & Koul (1965); Rampal (1967); Jyoti & Malhotra (1972); Mir (1979); Misra (1982); Qadri et al., (1983); Shekhar (1990); Kraus et al., (2000); Thapliyal (2002); Singh (2004); Kumar et al., (2006); Kume et al., (2006); Musa & Bhuiyan (2007); Narimatsu et al., (2007); Baruah & Borah (2008); Gandotra et al (2008) and Rahman & Haque (2008).
Material and Methods
About 50 specimens of Schizothorax richardsonii ranging from 21.0 to 32.9 cm of Total length were examined for the present study. The specimens, which were collected from April to June 2008, after capturing with the help of a cast net were brought to the laboratory and preserved in 10% formaline solution. The fishes were divided into Twelve length groups (Table 1). For the estimation of fecundity, a sample of 100 mg each from the anterior, middle and the posterior part of ovary were taken and the number of ovary in each sample was counted with the help of a binocular microscope. The fecundity was calculated as follows:
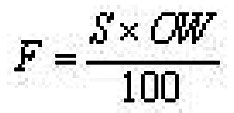
where
F= Fecundity,
S= Average number of ovary from 3 samples of 100 mg each and
OW= Total weight of ovary.
For measuring body weight and ovary weight a digital balance was used and for measuring diameter of ova occulometer was used. The relationships between different parameters were determined by the method of Least Square as follows:
Y = a +bX
where
Y= Dependent variable,
X= Independent variable,
a= Intercept and b= Slope
Results and Discussion
Relationship Between Fecundity and Fish Length
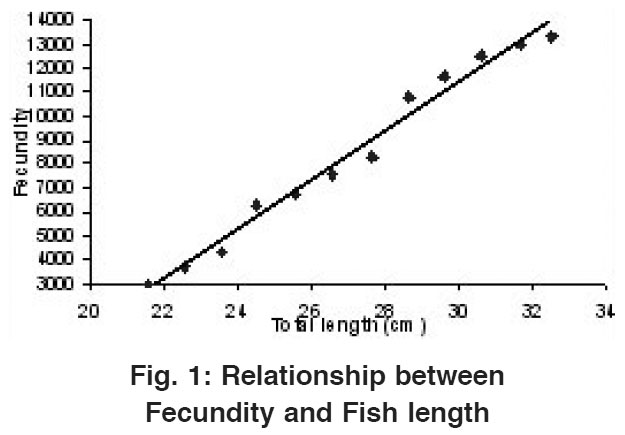 |
Figure 1: Relationship between Fecundity and Fish length Click here to view figure |
As depicted in Table 1 the total fecundity of the fish was found to increase from a minimum of 3032 in 21.54 cm average fish length (1st length group) to a maximum of 13321 in 32.51 cm average length (12th length group). A straight line was observed when Fecundity was drawn against fish length (Fig.1) which could be shown with the help of a Regression equation as: F= -19273.7 + 1023.91 FL. The value of correlation coefficient (r) was calculated to be 0.990.
Relationship Between Fecundity and Fish Weight
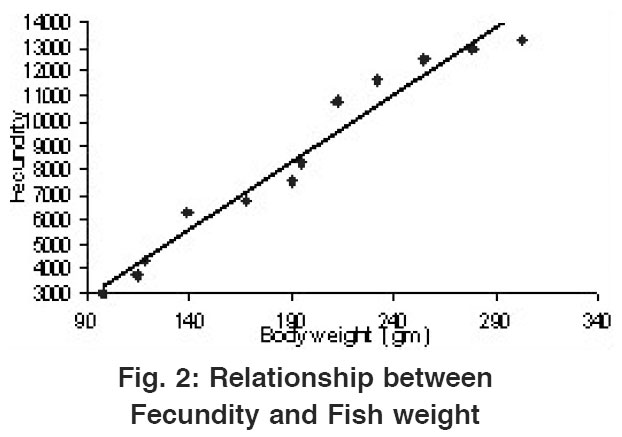 |
Figure 2: Relationship between Fecundity and Fish weight Click here to view figure |
Perusal of the Table 1 reveals that the lowest fecundity noted was 3032 in a fish weighing 96.98 gm of average weight (1st length group) and the highest fecundity was 13321 in a fish weighing 301.98 gm average weight (12th length group). A straight line was observed when Fecundity was drawn against fish weight (Fig.2) the Regression equation for which could be shown as: F= - 2052.68+54.74 FW. The value of correlation coefficient (r) was calculated to be r= 0.981.
Relationship Between Fecundity and Ovary Length
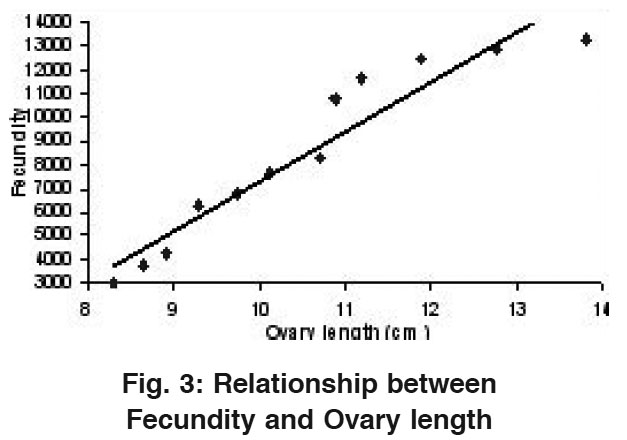 |
Figure 3: Relationship between Fecundity and Ovary length Click here to view figure |
As shown in Table 1 the total fecundity of the fish was found to increase from a minimum of 3032 in the fish having 8.29 cm average length of ovary (1st length group) to a maximum of 13321 in 13.80 cm ovary length (12th length group). A straight line was observed when Fecundity was drawn against Ovary length (Fig.3) which could be shown with the help of a Regression equation as: F= - 13725.1 + 2108.52 OL. The value of correlation coefficient (r) was calculated to be 0.955.
Relationship Between Fecundity and Ovary Weight
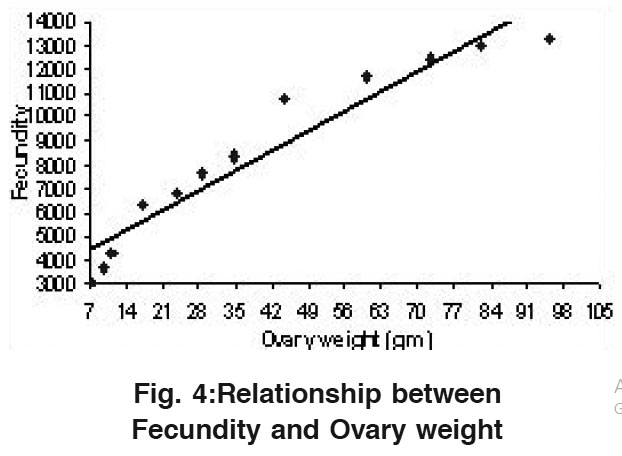 |
Figure 4:Relationship between Fecundity and Ovary weight Click here to view figure |
The average fecundity ranged from a minimum value of 3032 in a fish ovary having 7.45 gm of average weight (1st length group) and the highest fecundity was 13321 in a fish ovary having 95.21 gm average weight (12th length group) as shown in Table 1. A straight line was observed when Fecundity was drawn against ovary weight (Fig.4) which could be shown with the help of a Regression equation as: F= 3644.31+118.55 OW. The value of correlation coefficient (r) was calculated to be r= 0.959.
Relationship Between Fecundity and Ova Diameter
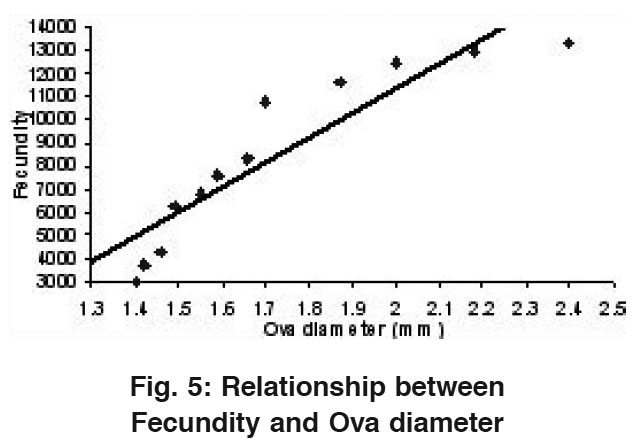 |
Figure 5: Relationship between Fecundity and Ovary diameter Click here to view figure |
The average fecundity ranged from a minimum value of 3032 in a fish having 1.4 mm of average ova diameter (1st length group) and the highest fecundity was 13321 in a fish having 2.4 mm of average ova diameter (12th length group) as shown in Table 1. A straight line was observed when Fecundity was drawn against ova diameter (Fig.5) which could be shown with the help of a Regression equation as: F= -9926.42 + 10641.46 OD. The value of correlation coefficient (r) was calculated to be r= 0.915.
Relationship Between Ovary Length and Fish Length
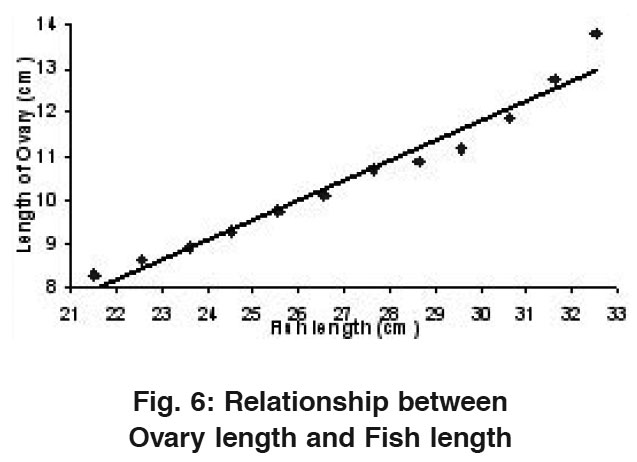 |
Figure 6: Relationship between Ovary length and Fish length ​​​​​​​Click here to view figure |
The average length of ovary ranged from a minimum value of 8.29 cm in 21.54 cm average fish length (1st length group) to a maximum value of 13.80 cm in 32.51 cm average length (12th length group). A straight line was observed when ovary length was drawn against fish length (Fig.6) which could be shown with the help of a Regression equation as: OL= -1.90 + 0.45 FL. The value of correlation coefficient (r) was calculated to be r= 0.980.
Relationship Between Ovary Weight and Fish Length
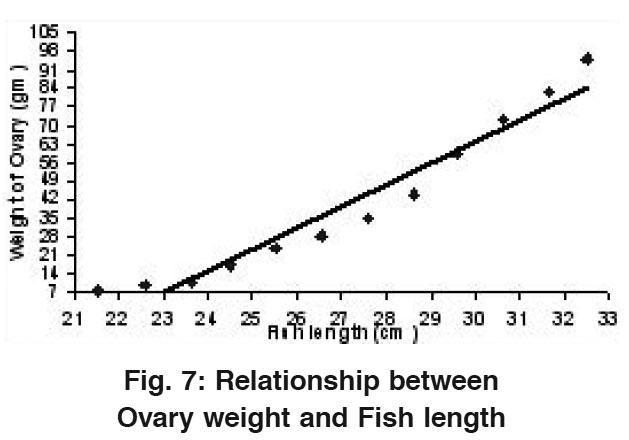 |
Figure 7: Relationship between Ovary weight and Fish length ​​​​​​​Click here to view figure |
The average weight of ovary ranged from a minimum value of 7.45 gm in 21.54 cm average fish length (1st length group) to a maximum value of 301.98 gm in 32.51 cm average length (12th length group). A straight line was observed when ovary weight was drawn against fish length (Fig.7) which could be shown with the help of a Regression equation as: OW= -179.23 + 8.11 FL. The value of correlation coefficient (r) was calculated to be r= 0.970.
Relationship Between Ovary Diameter and Fish Length:
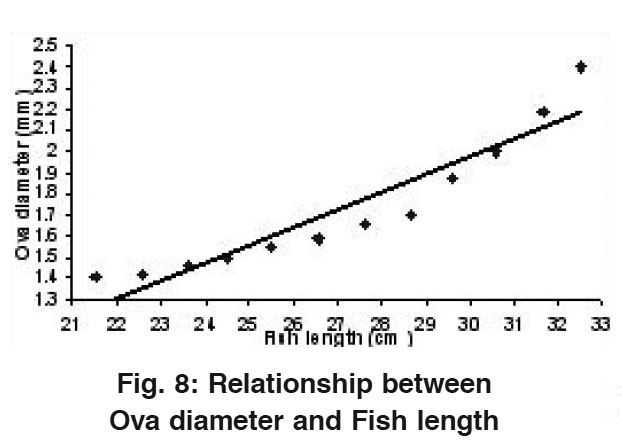 |
Figure 8: Relationship between Ovary diameter and Fish length ​​​​​​​​​​​​​​Click here to view figure |
The average diameter of ova ranged from a minimum value of 1.40 mm in 21.54 cm average fish length (1st length group) to a maximum value of 2.40 mm in 32.51 cm average length (12th length group). A straight line was observed when ova diameter was drawn against fish length (Fig.8) which could be shown with the help of a Regression equation as: OD= -0.53 + 0.08 FL. The value of correlation coefficient (r) was calculated to be r= 0.941.
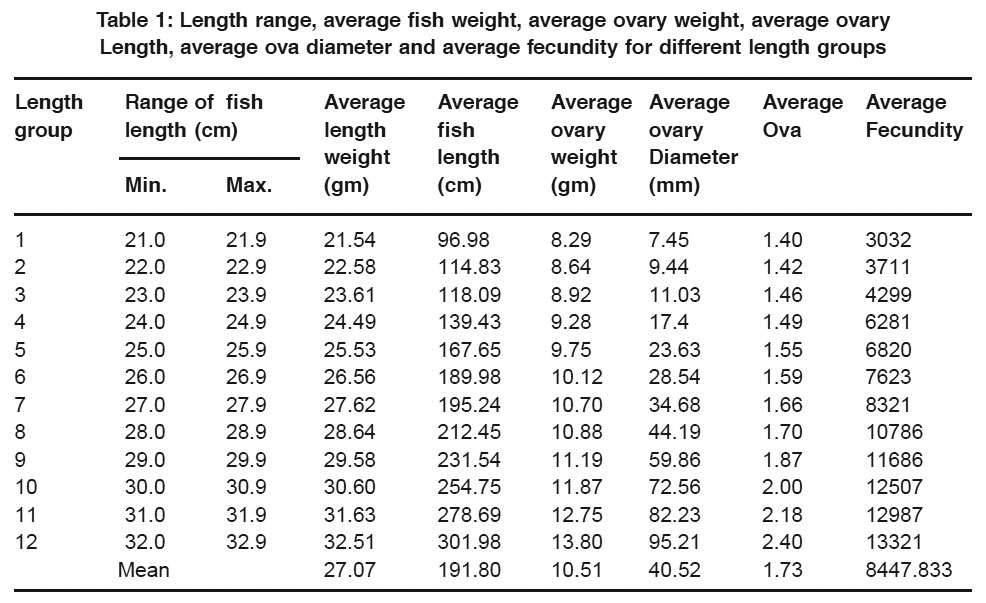 |
Table 1: Length range, average fish weight, average ovary weight, average ovary Length, average ova diameter and average fecundity for different length groups Click here to view table |
During the present studies, fecundity was found to vary from a minimum of 3032 in 21.54 cm average fish length to a maximum of 13321 in 32.51 cm average length with 8447.83 as mean value (Table1). The fecundity of Schizothorax richardsonii has already been calculated by Das & Koul (1965) from Kashmir to vary from a minimum of 2600 to a maximum of 16605 in the length groups ranging from 21.6 to 33.1 cm; Rampal (1967) from Kashmir to vary from 6720 to 27500 in the length groups ranging from 44.0 to 30.0 cm; Mir (1979) from Sindh Nallah in Kashmir to vary from 2598 to 27312 in the length groups ranging from 24.1 to 33.1 cm; Misra (1982) from river Alkananda from Garhwal Himalayas to vary from 3832 to 5310 in the length groups ranging from 35.5 to 53.1 cm; Qadri et al., (1983) from Sindh and Telbal Nallahs to vary from 2598 to 27846 in the length groups ranging from 22.0 to 47.5 cm and by Shekhar (1990) from Neeru Nallah in Bhadarwah to vary from 970 to 6035 in the length groups ranging from 17.0 to 34.9 cm.
Thorpe et al. (1984) and El-sarafy (1981) have advocated that egg production varies among individuals and populations of fish species due to differences in age and size. According to Kraus et al. (2000) the fecundity of individual fish of the same size drawn from the same spawning population within the same spawning season shows considerable deviation due to differential growth of gonads. According to Nikolsky (1963), the food consumed by the fish plays an important role not only in the fecundity but also the quality of the egg and their fertilization. Individual physiology of the fish and their surroundings may be the controlling factors for variation in Fecundity among fishes of same size (Hossain et al., 1997).
The present findings are in line with Das & Koul (1965) but differ from with the findings of other above mentioned authors which may be due to the difference in the length groups under study and also due to different geographical region having different climate, annual temperature and difference in the availability of food which affect the individual physiology of the fish.
Further, the statistical analysis of the present work revealed higher degree of correlation between fecundity and other parameters. However, the correlation between Fish length and Fecundity came out to be the highest (r=0.99) indicating the dependence of fecundity more on fish length than other body parameters. These results are in confirmation to the findings of Pimpicka (1981), Bhat and Dutt (1987) Singh et al., (1982); Thorpe et al., 1984; Babu & Nair (1985); Bhatt & Dutt (1987); Dobriyal and Singh (1987); Shekhar (1990); Rijnsdrop et al., (1991); Kraus et al., (2000); Thapliyal (2002), Singh (2004); Kumar et al., (2006); Kume et al. (2006), Narimatsu et al., (2007), Baruah and Borah (2008), Gandotra et al (2008) and Rahman and Haque (2008).
Thus, the present research findings clearly depict that the Schizothorax richardsonii inhabiting the lotic systems of Rajouri district is a medium fecund fish which breeds from April to June months shows the dependence of fecundity on the body length of fish.
References
- Babu, N. and N.B. Nair., Fecundity of Amblypharyngodon chakaiensis (Babu-Nair). Matsya, (1985) 11: 24-30.
- Bagenal, T.B., Fecundity (Part-I) In: Eggs and early life history. By T.B.Bagenal and Erich Braum: Methods of Assessment of Fish Production in Fresh waters. (Ed.) Ricker, W.E., IBP Hand Book No.3. Blackwell Scientific Publications, Oxford and Edinburgh (1968).
- Baruah, I. and B.C. Borah., Gonadal development and Fecundity of Gangetic mud eel Monopterus cuchia (Hamilton-Buchanan) in Assam. J.Inland Fish. Soc. India, (2008) 40(1): 69-74.
- Bhat, K.G. and N.H.G.Dutt., Fecundity in Puntius sarana (Ham.). In: Ichthyological notes. Matsya, (1987) 12-13: 208-217.
- Das, S.M. & B.L. Koul., The gross anatomy of gonads and fecundity of four fishes of Kashmir. Kash. Sci. (1965) 2(1-2): 64-76.
- Dobriyal, A.K. and H.R. Singh., The reproductive biology of a hill stream minor carp Barilius bendelisis (Ham) from Garhwal Himalaya, India. Vest cs. Spolec. Zool., (1987) 51: 1-10.
- El-Sarafy, S.S., Studies on some biological aspects of Clarias batrachus. Ph.D. Thesis, Calcutta University, Calcutta (1981).
- Gandotra, R., S.Ahmed, R.Shanker and S.Sagar., Fecundity in a hill stream minor carp Barilius vagra (Ham.) from Jhajjar stream, Jammu (J&K). J. Aqua. Biol., (2008) 23(1): 92-96.
- Hossain, M.A., S. Parween and M.A. Rahman., Fecundity of Nandus nandus (Ham.-Buch.) J. Biosc., (1997) 5: 305-307.
- Jyoti, M.K. & Y.R. Malhotra., Studies on fecundity of Schizothorax niger (Heckel) from Dal lake (Kashmir).Indian J. Expt. Biol. (1972) 10(1): 14-16.
- Kraus, G., A. Muller, K. Trella and F.W. Koster., Fecundity of Batic cod: Temporal and spatial variation. J. Fish. Biol., (2000) 56: 1327-1341.
- Kumar, K., K.L. Bisht, A.K. Dobriyal, H.K.Joshi, P.K. Bahuguna, S.Goswami, V.P. Balodi and A. Thapliyal., Fecundity and sex ratio in a rare hill stream fish Botia dayi (Hora) from Garhwal Himalaya, Uttaranchal. U.P. J. Zool., (2006) 26(3): 271-276.
- Kume, G., T. Horiguchi, A. Goto, H. Shirashi, Y. Shibata, Y. Morita and M. Shimizu., Seasonal distribution, age, growth and reproductive biology of marbled sole, Pleuronectes yokohamae in Tokyo Bay, Japan. Fish. Sci., 72: 289-298 (2006)
- Mir, S., Studies on the biology of Oreinus plagiostomus (Mc clleland). Ph.D. thesis, Kashmir University, Kashmir (1979).
- Misra, M., Studies on fishery biology of S. richardsonii (Gray), an economically important food fish of Garhwal Himalayas. D.Phil.thesis, Garhwal University, Srinagar (UP) (1982).
- Musa, A.S.M. & A. S. Bhuiyan., Fecundity of Mystus bleekeri (Day, 1877) from the river Padma near Rajshahi city. Turkish Journal of Fisheries and Aquatic Sciences, (2007) 7: 161-162.
- Narimatsu, Y., A. Yamanobe and M. Takahashi., Reproductive cycle, age and body size at maturity and fecundity of female willowy flounder Tanakius kitaharai. Fisheries Science, (2007) 73: 55-62.
- Nikolsky, G.V., The Ecology of fishes. London Academic Press, New York: 1-352 (1963).
- Nikolsky, G.V., Theory of fish population dynamics as the biological background for rational exploitation and management of fishery resources (Oliver & Boyd. Eds.). Edinburg: (1963) 323.
- Pimpicka, E., Fecundity of tench Tinca tinca in lake Patryki. Acta. Ichthyologica Et Piscatoria, (1981) 11(1): 3-20.
- Qadri, M.Y., S. Misra and Yousuf, A.R., Breeding biology of Schizothorax richardsonii (Gray & Hard).J.Indian Inst. Sci., (1983) 64(c): 73-81.
- Rahman, M.A. and M.M. Haque., Gonadal development of Gadusia chapra (Hamilton-Buchanan) from Rajdhala reservoir, Bangladesh. J.Inland Fish. Soc. India. (2008) 40(1): 10-15.
- Rampal, C., Studies on the life history of five Kashmir fishes together with fecundity and breeding habits. Ph.D. thesis, J&K University, Kashmir (1967).
- Rijnsdrop, A.D., N. Daun, F.A. Vanbeak and H.J.L. Hessen., Reproductive variability in North sea plaice, sole and cod. J. die Consiel Intl. pour Expl. de la Mer., (1991) 47: 352-375.
- Shekhar,C., Biology of Oreinus richardsonii (Grey & Hard) from Neeru-Nallah in Bhaderwah with a view to formulation of species conservation plan. Ph.D. Thesis, University of Jammu. Jammu (J&K) (1990).
- Singh, H.R., B. P. Nauriyal and A. K. Dobriyal., Fecundity of a hill stream minor carp Puntius chilinoides (McClelland) from Gharwal Himalaya. Proc. Indian. Acad. Sci., (1982) 91(5): 487-491.
- Singh, R., Some aspects of fish biology and parasitology of Noemacheilus botia (Ham.) from Khoh River. D. Phil. Thesis. H.N.B. Garhwal University (2004).
- Thapliyal, A., Some aspects of fish biology of the hill stream cat fish Pseudecheneis sulcatus (Mc Clelland) from Garhwal Himalaya, Uttaranchal. D.Phil. thesis, Garhwal University (2002).
- Thorpe, J.E., Miles, M.S. and Keau, D.S., Development rate, fecundity and egg size in Atlantic salmon, Salmo salar L. Aquaculture, (1984) 43: 299-305.






