Studies on ground water quality in Ghansawangi Taluka of Maharashtra
M.P. Gutte1 , K.N. Solunke1 and S.R. Mirgane1 *
1
Department of Chemistry,
Jalna Education Societys,
R.G. Bagdia Arts, S.B. Lakhotia Commerce and R. Bezonji Science, College,
Jalna,
431 203
India
DOI: http://dx.doi.org/10.12944/CWE.3.2.11
Physico - chemical studies of thirty two ground water (17 dug well and 15 bore well) samples from different parts of Ghansawangi town and taluka was carried out during the month of May 2007. The water quality parameters like temperature (T), pH, electrical conductivity (EC), total dissolved solids (TDS), total alkalinity (TA), total hardness (TH), chloride (Cl-), sulphate (SO42-), calcium (Ca2+), magnesium (Mg2+), sodium (Na+), potassium (K+), dissolved oxygen (DO) and turbidity (TUB) were studied and out come of the results were discussed. Physico - chemical studies of thirty two ground water (17 dug well and 15 bore well) samples from different parts of Ghansawangi town and taluka was carried out during the month of May 2007. The water quality parameters like temperature (T), pH, electrical conductivity (EC), total dissolved solids (TDS), total alkalinity (TA), total hardness (TH), chloride (Cl-), sulphate (SO42-), calcium (Ca2+), magnesium (Mg2+), sodium (Na+), potassium (K+), dissolved oxygen (DO) and turbidity (TUB) were studied and out come of the results were discussed
Copy the following to cite this article:
Gutte M.P, Solunke K.N, Mirgane S.R. Studies on ground water quality in Ghansawangi Taluka of Maharashtra. Curr World Environ 2008;3(2):273-278 DOI:http://dx.doi.org/10.12944/CWE.3.2.11
Copy the following to cite this URL:
Gutte M.P, Solunke K.N, Mirgane S.R. Studies on ground water quality in Ghansawangi Taluka of Maharashtra. Curr World Environ 2008;3(2):273-278. Available from: http://www.cwejournal.org/?p=839
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2008-09-28 |
|---|---|
| Accepted: | 2008-11-10 |
Introduction
Ghansawangi town is considered to be oldest and religious town in Jalna district of Marathwada region. A famous temple of lord ‘Narsinha’ is situated near Ghansawangi town. ‘Jamb Samarth’ village is a birth place of Samarth Ramdas Swami, Guru of Chatrapati Shivaji Maharaj, 25 Kms away from Ghansawangi, ‘Hazrat Sayeed Allauddin Saheb Darga’ is one of the important places of interest not only for the peoples of Marathwada but also other parts of India.Ghansawangi town is considered to be oldest and religious town in Jalna district of Marathwada region. A famous temple of lord ‘Narsinha’ is situated near Ghansawangi town. ‘Jamb Samarth’ village is a birth place of Samarth Ramdas Swami, Guru of Chatrapati Shivaji Maharaj, 25 Kms away from Ghansawangi, ‘Hazrat Sayeed Allauddin Saheb Darga’ is one of the important places of interest not only for the peoples of Marathwada but also other parts of India.
The residents of Ghansawangi town and taluka usually use water from dug well and bore well for drinking and domestic purposes. There is a huge variation in the concentration of different species due to factors like depth, different land, under ground water conditions, rain conditions etc. The present work attempts to evaluate the ground water quality in Ghansawangi taluka of Jalna district for potability. The residents of Ghansawangi town and taluka usually use water from dug well and bore well for drinking and domestic purposes. There is a huge variation in the concentration of different species due to factors like depth, different land, under ground water conditions, rain conditions etc. The present work attempts to evaluate the ground water quality in Ghansawangi taluka of Jalna district for potability.
Material used
In the present study thirty two ground water (17 dug well and 15 bore well) samples were collected from different parts of Ghansawangi town and taluka in brown glass bottles with necessary precautions and preserved as per the recommended procedures.¹ In the present study thirty two ground water (17 dug well and 15 bore well) samples were collected from different parts of Ghansawangi town and taluka in brown glass bottles with necessary precautions and preserved as per the recommended procedures.¹
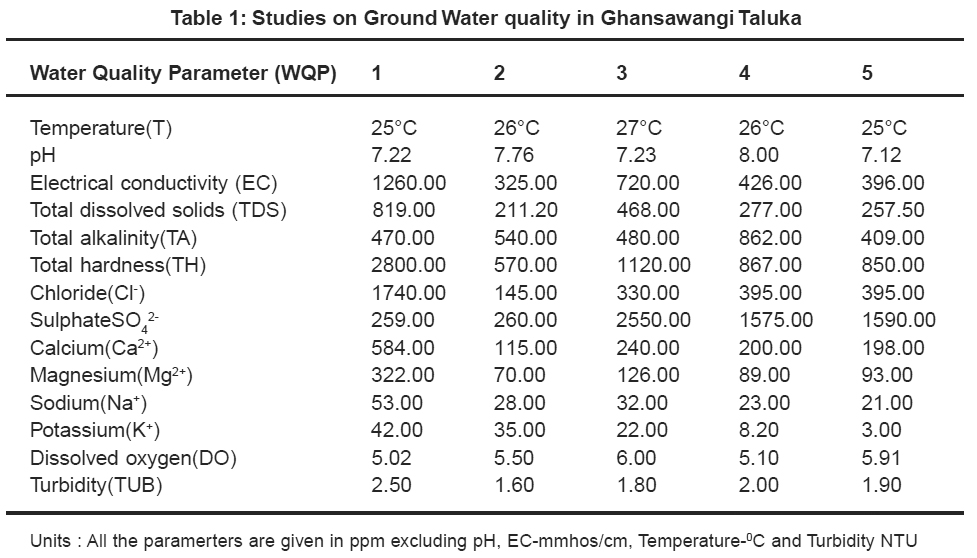 |
Table 1: Studies on Ground Water quality in Ghansawangi Taluka Click here to view table |
All the chemicals used were of AR grade, glass ware used were of ‘A’ grade. Double distilled CD. water was used through out the work to prepare standard solutions². All the chemicals used were of AR grade, glass ware used were of ‘A’ grade. Double distilled (DD) water was used through out the work to prepare standard solutions.2
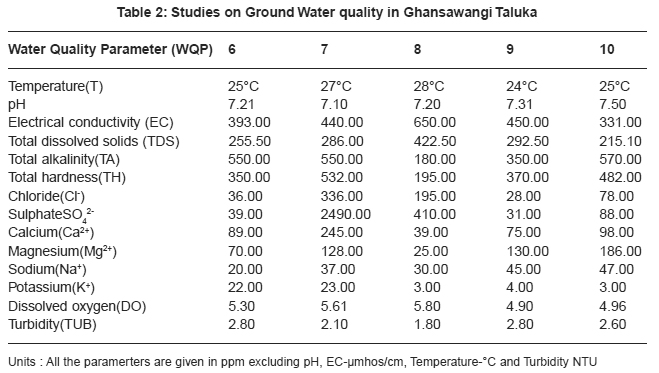 |
Table 2: Studies on Ground Water quality in Ghansawangi Taluka Click here to view table |
Methods
The water quality parameters (WQPs) considered for the examination in this study are temperature by precision thermometer (1100C), pH3 by digital pH meter (Model No. LI 613 Elico digital pH meter), electrical conductivity by using Elico digital conductivity meter (Model No. LICM 180),4 total dissolved solids by evaporation method at 105-1100C,5-6 total alkalinity by standard procedure,7 total hardness by complexometric titration method,8 chloride by argentometry,9 sulphate by nephleometry, calcium and magnesium by complexometre method, sodium and potassium by flame photometer (systronics, mediflame, Model No. 127, India), dissolved oxygen by winkler’s (Iodometric) method10 and turbidity by turbidimeter. The water quality parameters (WQPs) considered for the examination in this study are temperature by precision thermometer (1100C), pH3 by digital pH meter (Model No. LI 613 Elico digital pH meter), electrical conductivity by using Elico digital conductivity meter (Model No. LICM 180),4 total dissolved solids by evaporation method at 105-1100C5-6, total alkalinity by standard procedure,7 total hardness by complexometric titration method8, chloride by argentometry,9 sulphate by nephleometry, calcium and magnesium by complexometre method, sodium and potassium by flame photometer (systronics, mediflame, Model No. 127, India), dissolved oxygen by winkler’s (Iodometric) method10 and turbidity by turbidimeter.
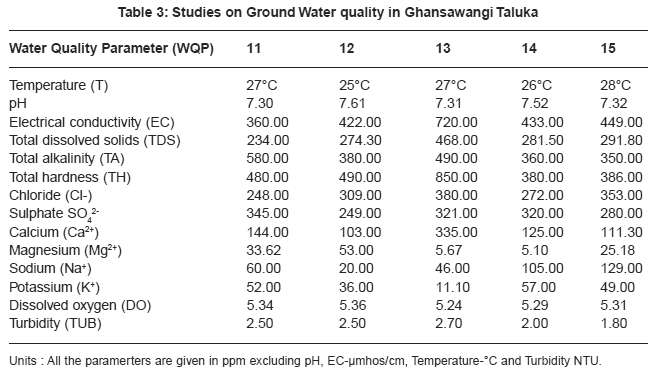 |
Table 3: Studies on Ground Water quality in Ghansawangi Taluka Click here to view table |
Results and Discussion
Thirty two ground water samples were collected from different parts of Ghansawangi town and taluka. The results indicates that the quality of ground water has wide variation which is reflected by the values of electrical conductivity, chloride, sulphate, calcium and magnesium etc.
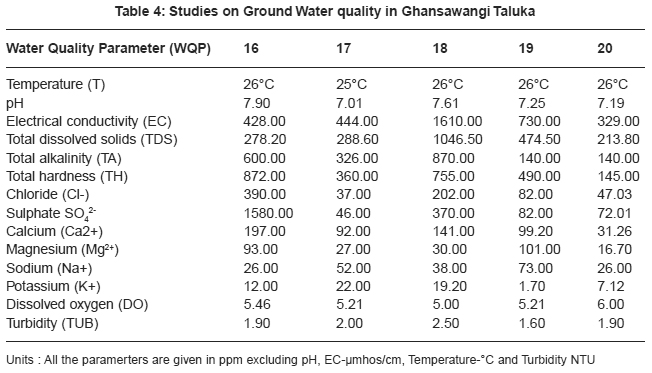 |
Table 4: Studies on Ground Water quality in Ghansawangi Taluka Click here to view table |
Thirty two ground water samples were collected from different parts of Ghansawangi town and taluka. The results indicates that the quality of ground water has wide variation which is reflected by the values of electrical conductivity, chloride, sulphate, calcium and magnesium etc.
pH acts as index to determine the extent of pollution, chemical and biological reactions are directly dependent upon the pH of water system. In the present study pH ranged from 7.01 to 8.00 which lies in the range prescribed by WHO11, electrical conductivity value, in present study ranged from 325 to 1610 mmhos/cm all were found to be well above the permissible limit and are quite unfit for drinking. pH acts as index to determine the extent of pollution, chemical and biological reactions are directly dependent upon the pH of water system. In the present study pH ranged from 7.01 to 8.00 which lies in the range prescribed by WHO11, electrical conductivity value, in present study ranged from 325 to 1610 mmhos/cm all were found to be well above the permissible limit and are quite unfit for drinking.
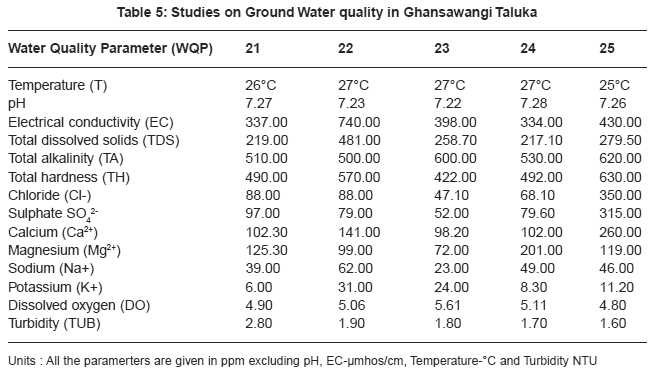 |
Table 5: Studies on Ground Water quality in Ghansawangi Taluka Click here to view table |
Drinking water quality is affected by the presence of soluble salts. Total dissolved solids (TDS) is an important parameter in drinking water quality standard. It develops a particular taste to the water and at higher concentration reduces its potability, plants are also severely affected by higher values of TDS in irrigation water. TDS value of study area ranges 211.20 to 1046.50 ppm. The high TDS level (7500 ppm) will result in the excessive scaling in water distribution system.12 Total alkalinity (TA) were found to be in the ranges 110 to 862 ppm. All samples are above the permissible limit prescribed by ICMR.13 The higher alkalinity of ground water owing to the presence of bicarbonates and trace amount of carbonate14 and hydroxide saltes.15 Water hardness is traditional measure of the capacity of water to reacts with soap. Hard water causes horrific effects in digestive systems moreover, the possibility of forming calcium oxalate crystals in urinary track has been ascertained. The hardness value of ground water in the present study area ranges from 145.00 to 2800.00 ppm.
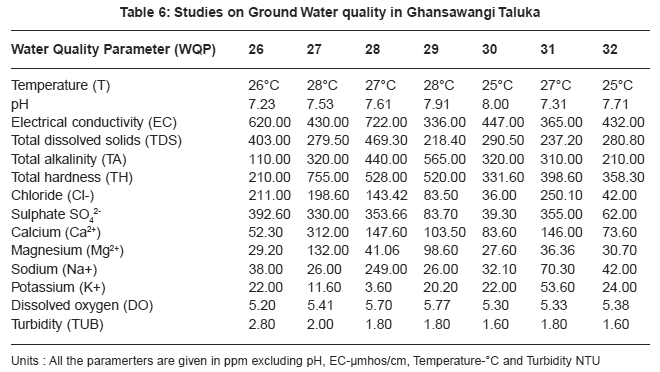 |
Table 6: Studies on Ground Water quality in Ghansawangi Taluka Click here to view table |
Chloride content were found to be ranging from 28.00 to 1740.00 ppm. Chloride in the twelve sites was found to be well above the permissible limit which may be due to the absence of proper drainage system in the study area. According to ISI permissible limit of sulphate concentration is 150 ppm. Beyond this limit, sulphate causes gastro-intestinal irritation and can have laxative effect in presence of magnesium and sodium. Sulphate ranges from 31.00 to 2550.00 ppm. In the present work calcium concentration varies from 31.26 to 584.00 ppm. High content of calcium may be due to leaching of soil deposit of limestone, dolomite, gypsum, gypsiferous materials, silicious sand into ground waters. Magnesium is an essential mineral for the living body. High concentration of magnesium causes nausea, muscular weakness and paralysis in human body, when it reaches upto the level of about 400 mg/L. In this area, magnesium concentration ranged from 5.10 to 322.00 ppm.
Sodium and potassium enters in drinking water from natural geological sources, detergents, domestic, industrial discharges and mining wastes. In the present work, sodium concentration varies from 20 to 249 ppm and potassium concentration varies from 1.70 to 57.00 ppm. Oxygen is dissolved in most waters in varying concentrations. Solubility of oxygen depends on temperature, pressure and salinity of water. It is essential to the life of fish and other aquatic organisms. In the present study dissolved oxygen ranges from 4.8 to 6.00 ppm. Turbidity is an important parameter for characterising water quality. In the present study turbidity varies from 1.6 to 2.8 NTU. These values are well below the permissible limit, 5 NTU as per WHO.
Acknowledgements
We are thankful to Principal Dr. R. S. Agrawal, Dr. S. M. Deshpande, Head of department, Jalna Education Society’s R. G. Bagdia Arts, S. B. Lakhotia Commerce and R. Bezonji Science, College, Jalna for providing necessary facilities and help for the present work.
References
-
American Society for Testing and Materials, Annual Book of-ASTM Standard, Part-23, ASTM-Phifadelphia, (1972)
-
A.l. Vogel, Text Book of Quantitative Inorganic Analysis 2nd edn., Longman & Green Co., London, 191, (1985).
-
R.G. Bates Determination, Theory of pH & Practice, 2nd Edn., Wiley, New York, (1973).
-
R.A. Robinson, and R.H. Stokes, Electrolytic Solutions Second Edn., Academic Press, New York, (1959).
-
N. Manivaskam, 'Physico-Chemical Examination of Water and Waste Water and Industrial Effluents', Pragati Prakashan, Meerut, India, (1983).
-
C.S. Howard, Determination of Total Dissolved Solid in Water Analysis, Introduction Engineering Chemistry, Anal. Ed., 5, (1933) 4.
-
Shell-Eitra, Encyclopedia of Industrial Ami Chemical Analysis 19, (2000) 1123.
-
apha, standard methods for the examination of water and waste water (16th Edn) Washington D.C., (1975)
-
I.M. Kolthoff, and V.A. Stenger, “Volumetric Analysis” - Second Edition Vol 2. Interscience Publishers, New York, (1947).
-
S. Hooda, and S. Kaur, “Laboratory Manual for Environmental Chemistry”, S. Chand and Company Ltd., (1999).
-
(WHO) World Health Organization, Guidelines for Drinking Water Quality, Recomendations of WHO; Geneva (1984 and 1996) 1, 1-130.
-
D.P. Tihansky, Water Resource, Res. (1974) 10 (2), 145-149.
-
ICMR Manual of Standards of Quality for Drinking Water Supplies’, Spl. Rep. S.No. 44, ICMR, New Delhi (India), (1975).
-
P Zuddas and F. Podda, App. Geochem, (2005) 20, 507-517.
-
N. Manivaskam, ‘Physico-Chemical Examination of Water and Waste Water and Industrial Effluents’, Pragati Prakashan, Meerut, India, (1983).






