Removal of metal ions from wastewater by using treated and untreated tree leaves as adsorbent
S.K. Wankhede1 * , U.V. Ladhe1 , B.D. Jagtap1 and P.R. Patil1
1
School of Envrionmental Science,
North Maharashtra University,
Jalgaon,
425 001
India
DOI: http://dx.doi.org/10.12944/CWE.3.2.16
Removal of metal ions such as chromium, iron and nickel from synthetic wastewater studied using tree leaves and activated carbon of tree leaves as adsorbent at ambeint temperature. Five different kinds of tree leave species and its activated carbons tested at the room temperature. The experiments were carreid out with 2gm of 0.60 mn to 1.60 mn leaf size in 200ml synthetic wastewaer containing 20-ppm metal ions. The initial pH of the synthetic wastewater was about 6. The experimental resuls indicate that highest removal rates were in activated carbon tree leave species that tree leaves. It was 85% chromium (Cr+6) by activated Neem, 76% iron (Fe+2) by activated Neem and Ber and 72% nickel (Ni+2) by activated Tea-waste.
Copy the following to cite this article:
Wankhede S.K, Ladhe U.V, Jagtap B.D, Patil P.R. Removal of metal ions from wastewater by using treated and untreated tree leaves as adsorbent. Curr World Environ 2008;3(2):301-306 DOI:http://dx.doi.org/10.12944/CWE.3.2.16
Copy the following to cite this URL:
Wankhede S.K, Ladhe U.V, Jagtap B.D, Patil P.R. Removal of metal ions from wastewater by using treated and untreated tree leaves as adsorbent. Curr World Environ 2008;3(2):301-306. Available from:http://www.cwejournal.org/?p=851
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2008-09-15 |
|---|---|
| Accepted: | 2008-10-20 |
Introduction
Heavy metal in wastewater have emerged as the focus of environment remediation efforts because of their toxicity and threat to industrialization and urbanization with new technology. Wastewater containing Ni, Cr, Fe originate from primarily metal industry particularly during plating, tannery operation. But their concentration and presence highly depends upon the type of method used for like ion-exchange, reverse osmosis, electrodialysis are costly and at the same time, methods like chemical precipitation, coagulation, flocculation etc. are less effective and has a disadvantage of sludge formation¹. However, such method is not preferred since sludge disposal problem. Adsorption is a sludge free process and had unergone many improvements. Besides these methods, now a day many scientists are attracted towards the adsorption is the process by which a solid adsorbent can attract a component from the aqueous phase to its surface and there by form an attachment via a physical or chemical bond, thus removing the component from the aqueous phase. Adsorption, which has been preferred over other process because of its cheapness and the high-quality treated effluent it produces.
Adsorption of Cr from Industrial effluents using agricultural waste², Ni from electroplating Industry by agro waste carbon,³ the removal Ni ions in aqueous media using low cost adsorbent,4 the adsorption of Fe using low cost materials,5 the adsorption of Fe, Ni, Cr using maize cob carbon.6 Many reports have been appeared on the development of low-cost activated carbon adsorbent from cheaper and readily available material7-9 their high surface areas, microporus characters and surface chemical natures have made such activated carbons potential adsorbent for the removal of heavy metals from industrial wastewater. Among the several methods, adsorption is highly effective and comparatively cheap, hence in present investigation to develop inexpensive and effective metal ions (Cr+6, Fe+2 and Ni+2) adsorbents from plentiful sources of natural wastes (or by-products) such as tree leaves (Ber, Neem, Ruchkin, Teak and Tea-waste) and its activated carbon to offer these adsorbents as replacements for existing commercial materials.
Material and Methods
Preparation of Adsorbent
The leaves of Teak (Verbenaceae), Ber (Zizipus amaranthus), Neem (Azardicita indica), Ruchkin (Calotropis) from twigs and Tea-waste from canteen were gathered into clean plastic bags. Washed with distil water and laid flat on clean table on dry. Dry leaves were grounded with mixer. After grounded, the leaf particle were sieved and stored into plastic bag is sieved size (0.60 - 1.60 mm), and ready for use.
 |
Table 1: Different tree leave species (fresh) as adsorbent for metal ions from Synthesis wastewater Click here to view table |
Chemical activation using H2SO4 at moderate temperature produces a high surface area and high degree of micro-pososity.10 The materials were mixed in 1:1 weight ratio with concentration H2SO4 and allowed to soak for 24h at a room temperature. The samples were placed in an over and heated at 200°C. Where they were held for 24 hours after this the sample was allow to cool back to room temperature. Then the samples were washed with distilled water and soaked in 1% NaHCO3 solution to remove any remaining acid. The samples were washed with the distilled water until pH of the activated carbon reached 6, dried at 105°C for 5 hours.
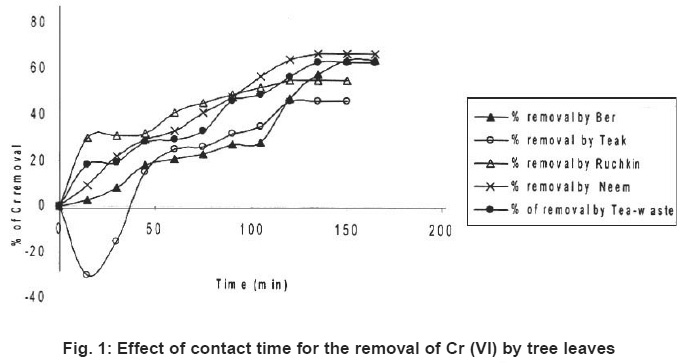 |
Figure 1: Effect of contact time for the removal of Cr (VI) by tree leaves Click here to view figure |
Synthetic Wastewater Preparation
All the chemicals were of analytical grade (Qualigens), Colorimeter (photoelectric AE-11m19942) were used determination of Cr, Fe and Ni, metal ions. The wastewater containing Cr, Fe and Ni was prepared in the laboratory by dissolving a known amount of salt i.e Cr, Fe and Ni in distilled water. All three solutions prepared about 20 ppm concentrated solution. The salt used as K2Cr2O7-7H2O, NiSO4-6H2O and FeSO4 7H2O.
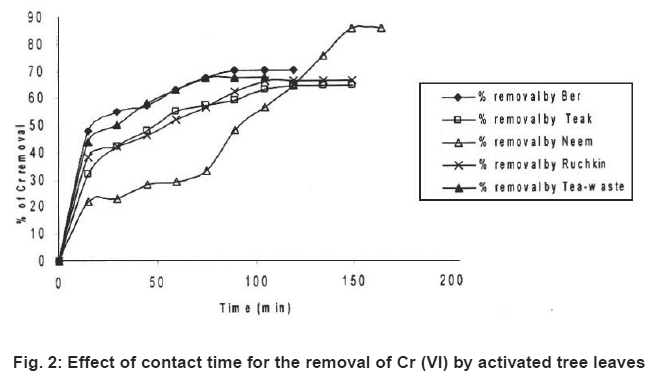 |
Figure 2: Effect of contact time for the removal of Cr (VI) by activated tree leaves Click here to view figure |
To understand the adsorption behavior a number of batch studies carried out like pH and contact time at constant of adsorbent dose for these studies. We prepare wastewater of various concentrtions of Cr, Fe and Ni. From stock solution of 20 ppm concentration and kept separately in a bottle then suitable dose (2gm) were added to the 200ml of wastewater. Shaking at 300 rpm for 2 to 3 hours at room temperature, so adequate contact between adsorbent and metal ions has maintained, equilibrates the system. The suspension is filter by centrifugation for 10 min at 4000 rpm and filtrate is analyzing to evaluate the final concentration of Cr, Fe, Ni metalss in treated wastewater by using calorimeter (Photoelectric calorimeter AE-11m, 99942). All the analysis carried out by standard method.¹¹
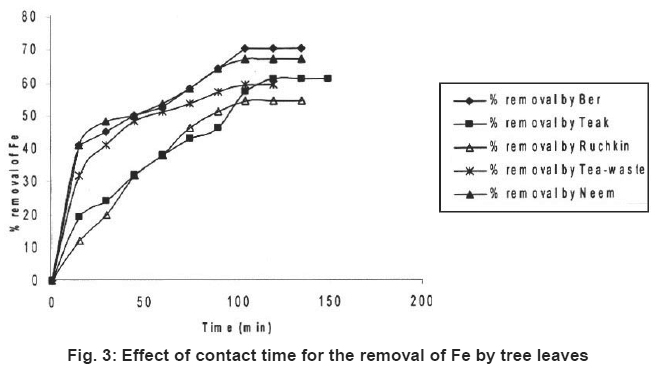 |
Figure 3: Effect of contact time for the removal of Fe by tree leaves Click here to view figure |
Results and Discussion
Adsorption on Tree Leaves (Fresh)
Table 1 represents the adsorption of three kinds of metal ions on five kinds of tree leaves. It reveals that different metal ion on the same tree leaf had different removal rate. At the same experimental conditions (Fe+2) and Cr (Cr+6) had highest removal rate. Ni+2 had lowest removal rate except Tea-waste. It has been observed that the highest removal rate for Cr+6 (67%), Fe+2 (70%) and Ni+2 (63%).
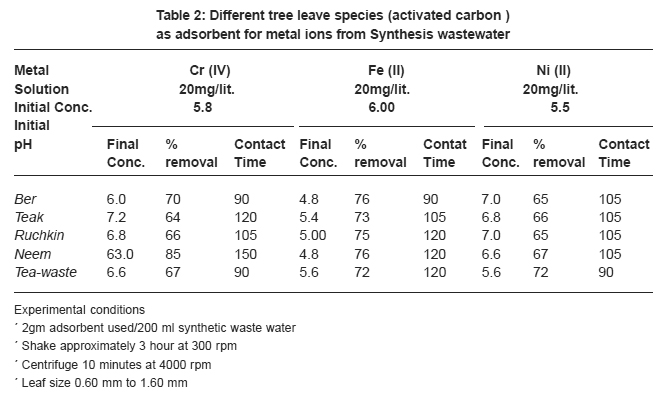 |
Table 2: Different tree leave species (activated carbon) as adsorbent for metal ions from Synthesis wastewater Click here to view table |
For the same metal ion, different tree leaves had different removal rates. In the tested leaf group Neem (67%) and Ber (64%) showed highest removal rates for chromium (Cr+6). Ber (70%) and Neem (67%) also showed higher removal rate for ions (Fe+2). The highest removal rates for Nickel ion (Ni+2) Tea-waste (63%) and Teak leave species (58%).
Adsorption Experiments
The experiments were carried in the batch mode for the measurements of adsorption capacities. The bottles with 250mL capacity were filled with wastewaer and adsorbent. The bottles were shaken for a predetermined period at room temperature in a reciprocating shaker. The separation of the adsorbents and solution was carried out by centrifugation. The solution were analyzed by using calorimeter.
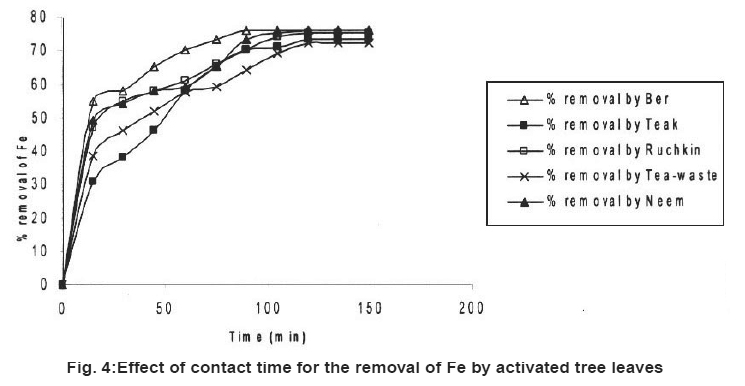 |
Figure 4: Effect of contact time for the removal of Fe by activated tree leaves Click here to view figure |
Adsorption on activated carbon of tree leaves
Table 2 also showed the results of adsorption of metal ion, Cr+6, Fe+2 and Ni2+ on the five kinds of activated carbon of tree leave species. The experiments were conducted under the same conditions as in the adsorption by tree leaves. The experimental results are tabulated in Table 2. It reveals that at the same experimental conditions (Cr+6), (Fe+2) and (Ni+2) had highest removal rate Cr+6 (85%), Fe+2 (76%) and Ni2+ (72%).
In the tested activated carbon of leaf group Neem showed highest removal rate for Cr+6, Neem and Ber showed highest removal rate for Fe+2 and the highest removal rate for Ni2+ ion Tea-waste.
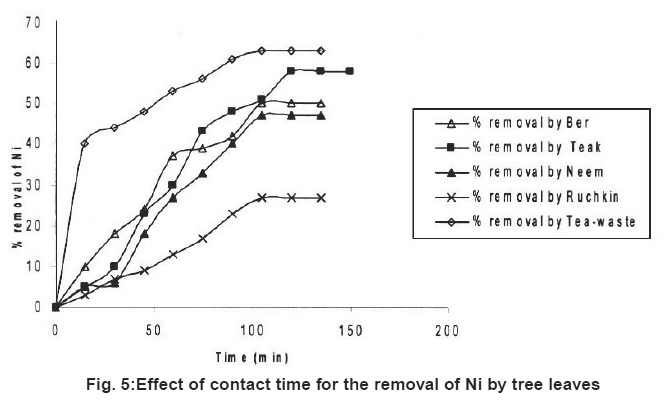 |
Figure 5: Effect of contact time for the removal of Ni by tree leaves Click here to view figure |
In comparison with the adsorption of metal ions from wastewater by activated tree leaves, did not showed supreme results. The best removal rate for Cr (VI) was by activated Neem leaves (85%) which are higher than Neem leaves (67%) under the same test conditions. Activated Ber removed (70%) and Tea-waste removed (67%) of Cr (VI), which is close results as tree leaves. i.e. (64%) and (63%) respectively. The best removal rate for Fe2+ was by activated Ber and Neem (76%) and activated Ruchkin (75%) which is higher than Ber tree leaves (70%), Neem tree leaves (67%) and Ruchkin (55%). The best removal rate for Ni2+ activated Tea-waste (72%) while other studied activated species were in the range (65-67%), which is higher than tree leave species.
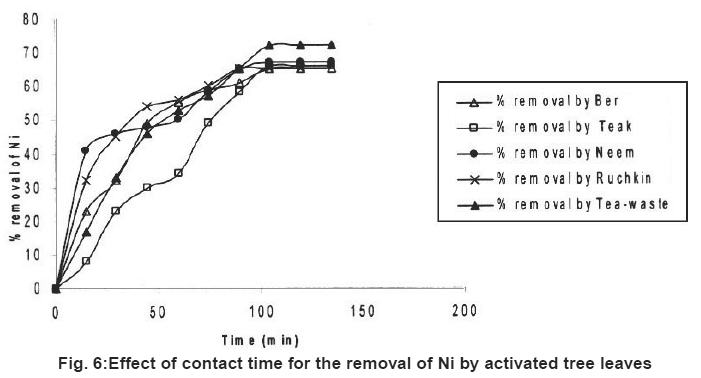 |
Figure 6: Effect of contact time for the removal of Ni by activated tree leaves Click here to view figure |
Influence of Contact Time on Adsorption
Preliminary experiments showed that teh adsorption of metal ions by leves reached equilibrium in more than 100 minutes (Fig. 1-6). The experimental resuls of kinetic study of chromium ion (Figs. 1-2), iron (Figs. 3-4) and nickel ion (Figs. 5-6) from synthetic waste water containing 20 ppm metal ions with Ber, Teak, Ruchkin, Neem, Tea-waste respectivley. The experiments were carried out under conidition of room temperature, with 2 gm of 0.60 mm to 1.60 mm leaf size in 200 ml of synthetic wastewater. The initial pH for synthetic wastewater was about 6. (Figs. 1-6). Reveals that the percentage of removal metal ions (Cr+6, Fe+2 and Ni2+) increases with an increase contact time at fixed absorption dose and concentration. Fig. 1 showed that the contact time used for all studied metal ions reveals that negative deviation by Teak leaves upto 40 minutes. This is because of may be due to small traces of chromium content in the leaves. The expeiments showed that hte removal rate occur quickly, seemly reaching equilibrium within the first hundred minutes of adsorption.
Conclusion
- Tree leaves can be used in the wastewater treatment process for the removal of metal ions.
- The adsorption of the metal ions on tree leaves reached equilibrium more than 100 minutes.
- The adsorption of different metal ions on the same tree leaf had different removal rate, chromium (Cr+6) had the highest removal rate, nickel (Ni++) had lowest removal rate.
- For the same metal ion, different tree leaves had different removal rate Ber and Neem showed highest removal rate for chromium (Cr+6). Tea-waste showed the highest removals rates for Nickel ion (Ni++) and Neem and Ber leaves showed that highest removal rate for Iron (Fe++).
References
-
Namasivayam C, Ranganathan, K., Removal of Cd (II) from wastewater by adsorption on "waste" Fe (III)/Cr (III) hydroxide. Wat. Res. (1994) 29: 1737.
-
Chand, Agarwal, V.K. and Kumar, P., Removal of Hexavalent Chromium from waste water by adsorption, Ind. J. Env. Health, (1994) 36(3): 151.
-
Batia Verma and N.P Shukla, Removal of Ni2+ from electroplaing industry effluent by agrowaste carbon, Ind. J. Env. Health., (2000) 42(4): 145.
-
M. Jitendra, R. Kulkarni and V.S. Shrivastava, Removal of Ni ion in aqueous medium using low cost adsorbent, J. Ind. Poll. Control, (2001) 12(2): 289-295.
-
L. Uzun and F. Guzel, Adsorption of some heave metal ions from aqueous soultion by activated carbon and comparison of percent Adsorption results of activated carbon with those of some other Adsorbents. Turk. J. Chem. (2000) 24: 91.
-
G. Sevakumari, M. Murugesan, S. Pattabi and M. Sathishkumar, Treatment of Electroplating industry Effluent using Maize cob Carbon, Bull. Environ. Contam. Toxicology. (2002) 69: 195.
-
Babel, S. and Kurniawan, T.A., Lowest adsorbent for heavy metal uptake form contaminated water: a review J. Hazardous matter, (1999) 97: 219.
-
Bailey, S.E., Olin, T.J., Bricka, R.M. and Adrian D.D., A review of potentially low cost sorbents for heavy mtal, Water Res., (1999) 33: 2469.
-
Pollard S.I.T., Flower G.E., Sollars, C.I., and Perry R., Low cost adsorbents for waste and wastewater treatment. A review Sci. Total Environ. (1992) 116: 31.
-
Demirbas, E., Kobya, M., Oncel M.S. and Sencan S., Removal of Ni (II) from aqueous solution by adsorption on to hazelnut shell activated carbon equilibirum studies. Bioresour. Technol., (2002) 84: 291.
-
APHA, Standard Methods for the examination of water and wastewater, (20th Edition), Americal Public Health Association, New York (1996).






