Malachite green dye, removal from waste water by adsorption
N. Buvaneswari 1 and C. Kannan1
1
Department of Chemistry,
Periyar University,
Salem,
636 011
Tamil Nadu
India
DOI: http://dx.doi.org/10.12944/CWE.3.2.12
The organic dyes directly pollute the soil, water, plants and all living systems in the environment. In the present investigation, adsorption of brilliant green dye on silica from aqueous solution was studied. The dye adsorption mechanism was studied on the basic surface of silica. The adsorption parameters were optimized to maximum adsorption. The adsorption mechanism of brilliant green on silica proved that the silica acts as a very good adsorbent for the removal of brilliant green dye.
Copy the following to cite this article:
Buvaneswari N, Kannan C. Malachite green dye, removal from waste water by adsorption. Curr World Environ 2008;3(2):279-282 DOI:http://dx.doi.org/10.12944/CWE.3.2.12
Copy the following to cite this URL:
Buvaneswari N, Kannan C. Malachite green dye, removal from waste water by adsorption. Curr World Environ 2008;3(2):279-282. Available from: http://www.cwejournal.org/?p=841
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2008-06-10 |
|---|---|
| Accepted: | 2008-08-09 |
Introduction
Dye wastewater from textile, dyeing and dye manufacturing industries discharged into water bodies which affects the aquatic flora and fauna, and causes many water borne diseases.1 Since synthetic dyes are complex aromatic compounds, they are more stable and more difficult to biodegradation.2 Today there are more than 10,000 dyes available commercially.3 The annual production of dyes worldwide is around 7×105 tonnes, 5-10% of which are discharged into water bodies as effluents.4 Therefore, good waste treatment methods are necessary to save the environment from dye pollution. The conventional methods for treating dye-containing wastewater like coagulation, flocculation, oxidation, photochemical destruction, ion exchange and membrane filtration5 are costly and require some additional chemicals. Hence, these methods are not much suitable to treat organic dye effluents. The adsorption methods have been widely used for removal of colorants from wastewater. Adsorbents like activated carbon,6 natural zeolites,7 Sawdust,8 and agricultural wastes9 have been used for dye removal.
In this present work, adsorption of cationic Brilliant green (BG) has been studied using silica as an adsorbent. Adsorption of BG was already studied using bagasse fly ash,10 neem leaf powder.11 Hence, in the present investigation, the attempts are made to adsorb BG on silica for maximum adsorption without any pretreatment of silica.
Experimental
Adosrption Studies
The dye BG supplied by Merk India Ltd. was used for adsorption studies on silica. The structure of BG is given in scheme 1. BG - Molecular formula C27H34N2O4S, Molecular Weight 482.65, C.I. No. 42040 and λ max 628-632nm.
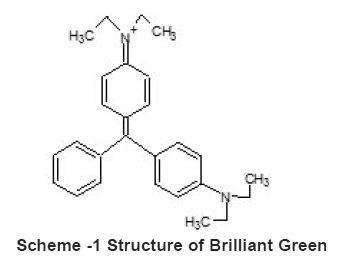 |
Scheme -1 Structure of Brilliant Green Click here to view scheme |
Adsorption Studies
The BG stock solution 1000ppm was prepared by dissolving 1g of dye in 1 litre of double distilled water in a standard measuring flask. The working solutions of the desired concentration were prepared by successive dilution of the stock solution. The dye concentration was analyzed by UV–Visible spectrophotometer (Elico- model-SL171). The dried silica (0.25g) added with 50ml of dye solution in 100ml conical flask separately. The solution stirred on magnetic stirrer (Remi-model-1MH) and at the end of the experiment, the solution was centrifuged off. The final concentration of the solution was measured by spectrophotometrically at λmax630nm. The contact time studied up to 1 hour to find out equilibrium adsorption. The adsorption process of BG on silica studied in the concentration range of 100-600mgdm-3. The temperature effect of BG studied in the range of 30-70oC. The adsorbent dosages studied in the range of 250-1000mg.
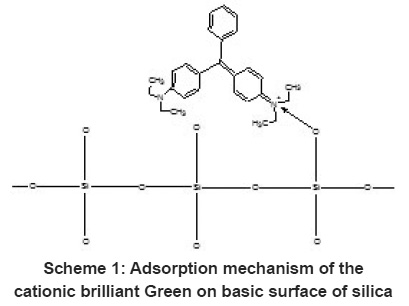 |
Scheme 1: Adsorption mechanism of the cationic brilliant Green on basic surface of silica Click here to view scheme |
Results and Discussion
Adsorption Studies
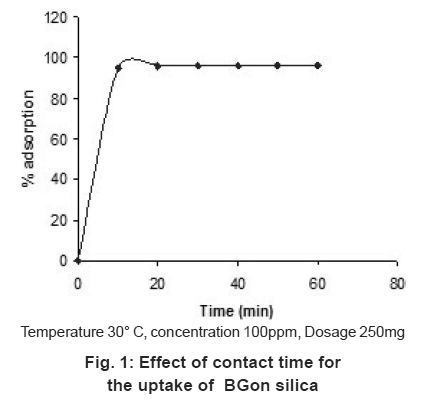 |
Figure 1: Effect of contact time for the uptake of BGon silica Click here to view figure |
Effect of Contact Time
The effect of contact time on the adsorption of BG was carried out at room temperature for 100ppm solution. Rapid adsorption observed within 20 minutes for the BG on silica and further increase of contact time not increase the adsorption. The removal of BG was 95%. The rapid adsorption may be due to the charge interaction between the cationic dye BG on the basic surface (silica) shown in Scheme 1 but the adsorption equilibrium was not established within 1hour may be due to the multilayer adsorption of BG in silica.
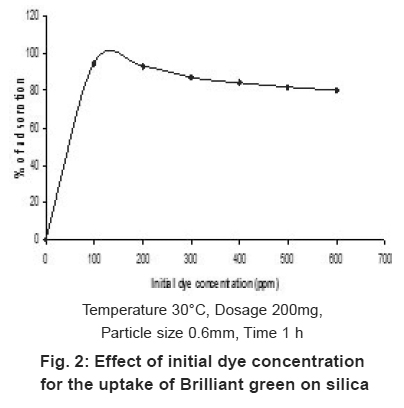 |
Figure 2: Effect of initial dye concentration for the uptake of Brilliant green on silica Click here to view figure |
Effect of Concentration of Dye
The effect of concentration of BG for maximum uptake was studied between the concentration ranges 100 to 600ppm. The studies were carried out at room temperature. The adsorption capacity of the dye was found to be decreases from 95% with increasing the BG concentration (Fig.2). The percentage of uptake decreases with increase of BG concentration was due to the saturation of active sites with BG molecule. Hence, adsorption decreases with increase of BG concentration.
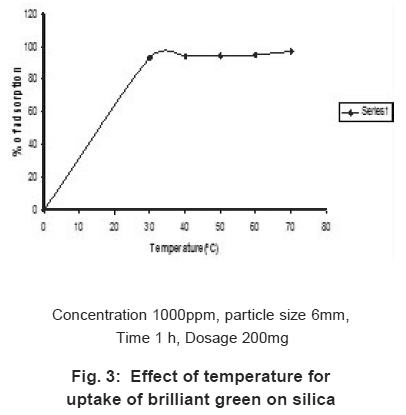 |
Figure 3: Effect of temperature for uptake of brilliant green on silica Click here to view figure |
Effect of Temperature
The effect of temperature for the removal of BG was studied for the concentration 500ppm of BG solution in the range of 30 to 70°C (Fig. 3). The adsorption increases 93% to 97% when the temperature increases from 30 to 70°C.This indicate that the adsorption is endothermic and chemisorption.
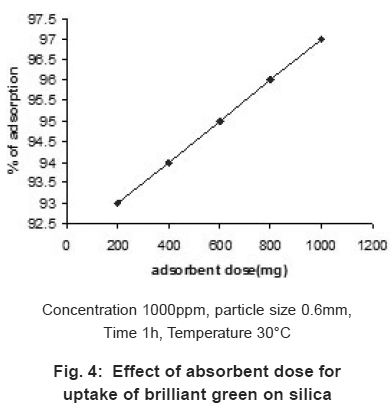 |
Figure 4: Effect of absorbent dose for uptake of brilliant green on silica Click here to view figure |
Effect of Dosage
The effect of adsorbents dosage (200-1000mg) for adsorption of BG was studied.
The percentage adsorption of the dye was found to be increases with increase of adsorbent dosage (Fig.4), since the increase of dosage increases the surface area and active sites for more adsorption of BG on sawdust. The adsorption capacity of BG was 180mg/g.
Conclusion
The BG adsorption equilibrium rapidly established within 20 minutes due to the chemical interaction between the cationic dye and basic silica surface. Adsorption not much increased with increase of temperature proved the rapid adsorption of BG at room temperature. The uptake of BG decreased with increase of concentration attributed to the saturation of active sites and surface area of the adsorbent. The BG removal increased with increase of adsorbent dosages due to the increase of active sites and surface area of the adsorbent. The adsorption mechanism of brilliant green on silica proved that the silica acts as a very good adsorbent for the removal of the azo dye brilliant green.
References
-
J.Gezechulska, A.W.Morawski; Applied CatalysisB: Environmental, (2002) 36: 45.
-
C.A. Fewson; Trends Biotechnol., 6: 148 (1988).
-
R.Gong, M.Li, C.Yang, Y.Suna, J.Chenb; J.Hazard mater., (2005) 21: 247.
-
C.Sudipta, C.Sandipan, P.C.Bishnu, R.D.Akil, K.G.Arun; J.Colloid Interface Sci., (2005) 288: 30.
-
Y.Önal, C.Akmil-Baºar, D.Eren, Ç.SarýcýÖzdemir, T.Depci; J.Hazard Mater., (2001) 128: 150.
-
Y.Fu, T.Viraraghavan; Bioresour Technol., (2001) 79: 251.
-
Y.G.Ko, U.S.Choi, J.S.Kim, Y.S.Park; Carbon, (2002) 40: 2661.
-
S.Wang, Z.H.Zhu; J.Hazard Mater., (2006) 136: 946.
-
S.Wang, H.Li, L.Xu; J.Colloid Interface Sci., (2006) 295: 71.
-
F.A. Batzias, D.K.Sidiras; Bioresour Technol., (2007) 98: 1208.
-
B.G.Prakash Kumar, K.Shivakamy, L.R.Miranda, M.Velan; J.Hazard Mater., (2006) 136: 922.






