Total solids occurring in various industries effluent water present in Durg district
Parminder Kaur1 *
1
Department of Chemistry,
Indira Gandhi Govt. College,
Vaishali nagar,Bhilai,
Durg,
490 023
India
DOI: http://dx.doi.org/10.12944/CWE.3.1.24
For pure water the hardness is very low because in it low dissolved ions. Water with no dissolved solids usually has flat taste, whereas water with more than 500mg/l tds has a disagreeably taste. In case of industrial effluent water in which many dissolved ions are their the hardness is very high and it will show many soluble matter in it. The hard water retards the cleaning action of soaps and detergents causing an expense in the form of extra work and cleaning agents. Furthermore, if the hard water is heated it deposits a hard scale on heating coils, cooking utensils, and other equipment with a consequent waste of fuel. The scale formed by the hard water coats the inside of distribution system piping, which can eventually cause significant reduction in its water carrying capacity.
Copy the following to cite this article:
Kaur P. Total solids occurring in various industries effluent water present in Durg district. Curr World Environ 2008;3(1):157-160 DOI:http://dx.doi.org/10.12944/CWE.3.1.24
Copy the following to cite this URL:
Kaur P. Total solids occurring in various industries effluent water present in Durg district. Curr World Environ 2008;3(1):157-160. Available from: http://www.cwejournal.org/?p=793
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2008-03-17 |
|---|---|
| Accepted: | 2008-05-02 |
Introduction
Total dissolved solids also called total filterable residue is a measure of the water C™s content of various dissolved materials. Water with no dissolved solids usually has a flat taste, whereas water with more than 500 mg/l TDS usually has a disagreeably strong taste. Depending on the chemical nature f the dissolved solids, reverse osmosis or lime soda softening may be used to reduce TDS content. Primary sources for TDS in receiving waters are agricultural runoff, leaching of soil contamination and point source water pollution discharge from industrial or sewage treatment plants. The most common chemical constituents are calcium, phosphates, nitrates, sodium, potassium and chloride, which are found in nutrient runoff, general stormwater runoff and runoff from snowy climates where road de-icing salts are applied. The chemicals may be cations, anions, molecules or agglomerations on the order of 1000 or fewer molecules, so long as a soluble micro-granule is formed. More exotic and harmful elements of TDS are pesticides arising from surface runoff. Certain naturally occurring total dissolved solids arise from the weathering and dissolution of rocks and soils.
High TDS levels generally indicate hard water, which can cause scale buildup in pipes, valves and filters, reducing performance and adding to system maintenance costs. These effects can be seen in aquariums, spas, swimming pools and reverse osmosis water treatment systems. Typically, in these applications, total dissolved solids are tested frequently and filtration membranes checked in order to prevent adverse effects. Dissolved solids, as a whole, are about 40 percent organic and 60 percent inorganic.
Total solids, as the term implies, includes all of the solid constituents of a wastewater. Total solids are the total of the organic and inorganic solids or the total of the suspended and dissolved solids. The principal application of TDS is in the study of water quality for streams, rivers and lakes, although TDS is generally considered not as a primary pollutant (e.g. it is not deemed to be associated with health effects), but it is rather used as an indication of aesthetic characteristics of drinking water and as an aggregate indicator of presence of a broad array of chemical contaminants. Since the threshold of acceptable aesthetic criteria for human drinking water is 500 mg/l, there is no general concern for cancer as most humans will reject consuming drinking water due to odor, taste and color at a level much lower than is required for harm. However,exposed to high TDS levels from human interference (and, rarely, natural occurrences). A number of studies have been conducted and indicate various species’ reactions range from intolerance to outright toxicity due to elevated TDS. Obviously, the numerical results must be interpreted cautiously, since true toxicity outcomes will relate to specific chemical constituents. Nevertheless, some numerical information is a useful guide to the nature of risks in exposing aquatic organisms or terrestrial animals to high TDS levels. Most aquatic ecosystems involving mixed fish fauna can tolerate TDS levels of 1000 mg/l.
Research has shown that exposure to TDS is compounded in toxicity when other stressors are present, such as abnormal pH, high turbidity or reduced dissolved oxygen with the latter stressor acting only in the case of animalia.
Industrial effluent contains considerable amount of undissolved matter. Determination of dissolved and undissolved matter is made with filtered and unfiltered portions of samples. Undissolved matter is referred to as suspended solids.
Material and Methods
Gravimetric methods are the most accurate and involve evaporating the liquid solvent to leave a residue, which can subsequently be weighed with a precision analytical balance (normally capable of .0001 gram accuracy). This method is generally the best, although it is time consuming and leads to inaccuracies if a high proportion of the TDS consists of low boiling point organic evaporate along with the water. In the most common circumstances inorganic salts comprise the great majority of TDS, and gravimetric methods are appropriate.For evaporation, dishes of 250ml capacity, are employed. For industrial effluent samples porcelain dishes were used.
-
Place the evaporating dish containing the 100ml of effluent water in muffle furnace at temp.103°C for one hour. So, the water evaporated and the solid residue of yellowish white color obtained.
-
Cool the dish-containing residue, put in desiccators to obtain room temperature and to absorb moisture in desiccator.
-
Weigh the dish. Then deduct the weight of empty dish in it to obtain the total dissolved solid in effluent water.
Total Suspended Solids
Porcelain dish take effluent sample dry in an oven at the same temperature intended for the residue cool in a desiccator and weigh. Place it on a water bath and measure into it 100 ml of well-mixed sample and further dry it at 105 °c for 1 hr. in over cool and weigh
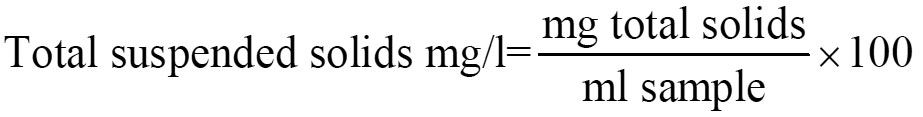
Result = Total solids on drying at 105 0c in terms of mg/l.
Total Fixed Solids
Keep the above dish in muffle furnace at 550 °c for 1 hr. any organic matter and volatile substance present will be volatilized at this temperature and the remaining is the total fixed solids.
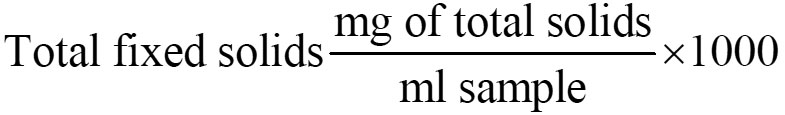
Total Volatile Solids mg/l
Total solids mg/l Total fixed solids mg/l
Total Dissolved Solids
Filtered sample is evaporated to dryness at 180 °C. Increase in weight of dish is equal to total dissolved solid.
Result
Total dissolved solids 180°c is in terms of mg/l.
Fixed dissolved solids-Ignite the above residue in muffle furnace at 550 °C for 1hr cool and weigh.
Result is in mg/l
Volatile dissolved solids mg/l=Total dissolved solids mg/l-Fixed dissolved solid mg/l.
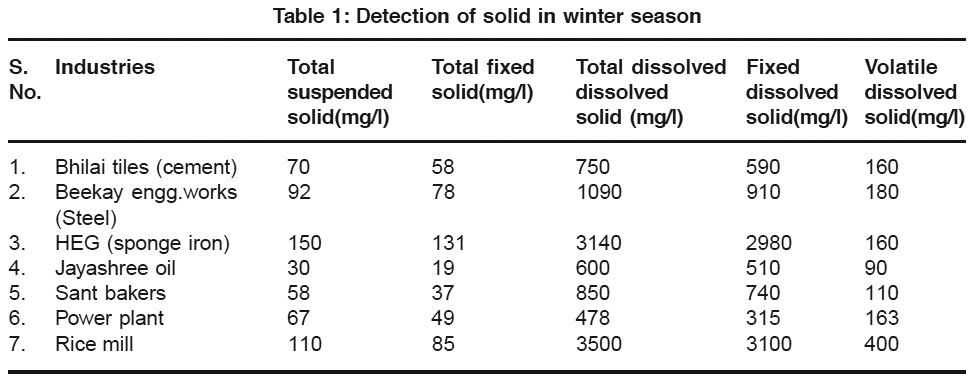 |
Table 1: Detection of solid in winter season Click here to view table |
Working Area
Chhattisgarh state is a newly formed state, situated by the side of Madhya Pradesh. In Chhattisgarh Durg is the one of the district where small scale industries of cement pipe making, sponge iron plant, rice mill, bakery, oil mill, power plant is present. Any liquid effluents, when discharged will eventually find its way into the hydrological cycles and thereby can have adverse effects on the ecosystems and eventually on the quality of water to the consumer. It is important to bear in mind that the small size of the island means that very quickly water sources can be adversely affected by effluent pollution.
They throw their industries effluent water coming from the industries into the stream, which pollute the water at the vicinity of that industry. People living nearby carry many types of diseases. The different industries of Durg Distt. namely Bhilai Tiles, Beekay engg. Works, Sant Bakers, Jayashree oil, HEG, Borai, Ecophane power plant, Venkatesh rice mill situated at Durg Distt were taken for the compliance monitoring of effluent water.
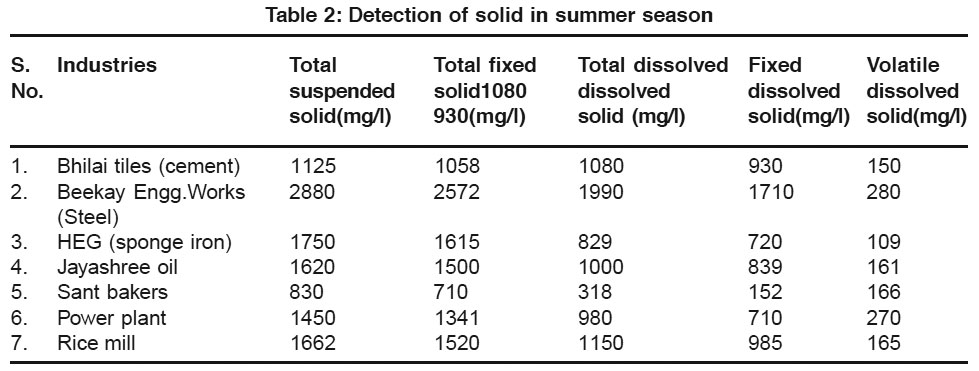 |
Table 2: Detection of solid in summer season Click here to view table |
Results and Discussion
In above table the volatile dissolved solids of different effluent water coming out from different industries situated in Durg district Chhattisgarh is present. It shows that the effluent water of HEG (sponge iron) Rice mill industry shows the maximum of total dissolved solid in winter season but Beekay Engg works show maximum total dissolved solid in summer season which form scales on the water surface when water was in stream. The effluent water of power plant and Jayashree oil industry shows the minimum amount of hardness in winter season but sant bakers show minimum TDS in summer season i.e. the water was somewhat pure and clear in tolerance limit. After observing the table it shows the decreasing order of loss of ignition of industries
In winter season from Rice mill (400), Beekay Engg. Works (180), Power plant (163), HEG (sponge iron plant) &Bhilai tiles (160), Sant Bakers (110), Jayashree oil (90).
In summer season Beekay Engg.works (280), Power plant (270), Sant Bakers (166), Rice mill (165), Jayashree oil (161), Bhilai Tiles150), HEG Sponge iron plant (109).
Depending upon the chemical nature of the dissolved solids, reverse osmosis or soda lime softening may be used to reduce TDS content. Total dissolved solids limitation present in water the acceptable limit is 500mg/l & cause of rejection is 1500mg/l. Tolerance limits for inland surface waters for irrigation is 2100mg/l.
An ISI standard for discharge of industrial effluents is 2100mg/l, which is useful for irrigation. It is said that if we can provide pure drinking water, the 98% disease can run away automatically from this country.
Water Quality Standard
The Quality of Water is Dependent on Total Solid.
|
Quality |
Total solids (ppm) |
|
Excellent |
175 |
|
Good |
175-525 |
|
Permissible |
525-1400 |
|
Doubtful |
1400-2100 |
|
Unsuitable |
>2100 |
The environmental protection agency recommends treatment when TDS concentration exceeds 500 ppm. The TDS concentration is considered as a secondary drinking water standard, which means that it is not a health hazard.
Environmental Impact
Suspended solids can clog fish gills, either killing them or reducing their growth rate. They also reduce light penetration. This reduces the ability of algae to produce food and oxygen. When this water collect in a reservoir the suspended sediment settles out in the bottom. This causes the water to become clear but sediment settles change the bottom. It kills bottom dwelling organisms, covering breeding area and smoother eggs.
TSS affects other parameters such as temperature and-dissolved oxygen. Because of the greater absorbency of the particulate matter the surface water becomes warmer and this tends to stabilize stratification. Suspended solids interfere with effective drinking treatment. It also interferes in recreational use like swimming. Poor visibility can be dangerous for swimming and diving.
Positive Impact on Environment
A positive effect of the presence of suspended solids in water is that toxic chemicals such as pesticide and metals tend to adsorb to them or become complexes with them, which makes the toxic less available to be absorbed by living organisms.
Acknowledgements
The author wishes to acknowledge the University Grants Commission, CRO, Bhopal for providing financial assistance. My sincere thanks to Principal, Dr.Kailash Sharma for continuous encouragement and support for doing my research work.
References
- Dickinson, D., C˜ The chemical analysis of waters,boiler and feed waters,sewage and effluents. C™Second edition,1950,Blackie & son,London.
- Teeroux, F.R., Eldridge, E.F. and Mallmann, W.L., Laboratory Manual for Chemical and Bacterial analysis of water and sewageC™-Third Edition 1943,Mcgraw Hill,New York.
- Manivasakam, N., Physico-chemical examination of water sewage and industrial effluentsC ™Fifth edition,2005,pragati Prakashan,Meerut.
- Water quality and total solids in relation to topography,www.grc.nasa.gov/www/k-12/ fenlewis/waterqualitysolids.htm
- Water Quality, www. advancedinspections. net/gpage3.html
- www.dissolvedoxygen andwaterquality.html






