The use of low cost and environment friendly materials for the removal of heavy metals from aqueous solutions
Nour El- Din T. Abdel-Ghani1 * and Ghadir. A. El-Chaghaby2
DOI: http://dx.doi.org/10.12944/CWE.3.1.05
The objective of this study was to evaluate the use of rice husk and Nile rose plant for their ability to remove Cr (III), Cu(II), Zn(II), Cd(II) and Pb(II) from their mixed aqueous solution. The effects of contact time, pH, initial metal concentration and amount of adsorbent on the adsorption process at room temperature 25 ± 2°C were studied. Batch adsorption studies showed that an equilibrium time of 90 min. was required for the adsorption of Cr (III), Cu(II), Zn(II), Cd (II) and Pb(II) on both investigated adsorbents. The maximum metal removal was found to be pH dependent . With an increase in the concentrations of these metals, their adsorption decreased on both of the adsorbents. The experimental data were best fitted to the Temkin isotherm model. Rice husk and Nile rose plant were found to be good metal adsorbents. A case study was also performed to examine the feasibility of using the investigated adsorbents for treating real electroplating wastewater.
Copy the following to cite this article:
Abdel-Ghani N.E.T, El-Chaghaby G.A. The use of low cost and environment friendly materials for the removal of heavy metals from aqueous solutions. Curr World Environ 2008;3(1):31-38 DOI:http://dx.doi.org/10.12944/CWE.3.1.05
Copy the following to cite this URL:
Abdel-Ghani N.E.T, El-Chaghaby G.A. The use of low cost and environment friendly materials for the removal of heavy metals from aqueous solutions. Curr World Environ 2008;3(1):31-38. Available from: http://www.cwejournal.org/?p=736
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2008-02-17 |
|---|---|
| Accepted: | 2008-05-09 |
Introduction
Heavy metals are nowadays among the most important pollutants in source and treated water, some of these are cumulative poisons capable of being assimilated, stored and concentrated by organisms that are exposed to low concentration of these substances for long periods or repeatedly for short periods.¹ Heavy metal removal from aqueous solutions has been commonly carried out by several processes: chemical precipitation, solvent extraction, ion-exchange, reverse osmosis or adsorption.²,³
Among these processes, the adsorption by a suitable adsorbent can be an effective technique for the removal of heavy metals from wastewater.4-6 Over the recent years, a growing research interest has been prompted into the production of low cost alternatives to the commonly used expensive adsorbents. In our previous work we investigated the removal of metal ions from their aqueous solutions using many low cost adsorbents.7-8
Rice milling generates a by product known as rice husk. This surrounds the paddy grain. During milling of paddy about 78% of weight is received as rice, broken rice and bran. Rest 22% of the weight of paddy is received as husk. This husk contains about 75% organic volatile matter and the balance 2 % of the weight of this husk is converted into ash during the firing process, is known as rice husk ash (RHA). So for every 1000 kg of paddy milled , about 220 kg (22%) of husk is produced , and when this husk is burnt in the boilers , about 55 kg (25%) of RHA is generated.
Nile rose plant (water hyacinth plant). The water hyacinth Eichhornia crassipes is considered the world’s worst invasive aquatic weed. In Africa, it was first recorded in the 1890s from the River Nile in Egypt, but since then become widespread throughout the continent. The plant thrives in still and slow-moving water-bodies that have become nutrient-enriched through eutrophication, and dense mats of water hyacinth now blanket many of Africa’s dams, lakes, rivers and canals.This plant causes water loss through evaporation, obstruction of navigation and fishing, and blockage of irrigation and drainage systems.
 |
Figure 1: Equilibrium time for the adsorption of Cr (II), Cu (II), Zn(II), Cd (II) and Pb (II) on rice husk Click here to view figure |
The present study was undertaken to evaluate the effectiveness of both rice husk and Nile rose plant in the removal of Cr3+, Cu2+, Zn2+, Cd2+ and Pb2+ from a mixed metal ions solution by adsorption. Laboratory batch experiment and isotherm studies were conducted to determine the adsorption efficiency of husk and Nile rose plant. The effect of contact time, pH, initial concentration of adsorbate and adsorbent dosage on adsorption were studied. A case study was also performed to examine the feasibility of using the investigated adsorbents for treating real electrplating wastewater.
Material and Methods
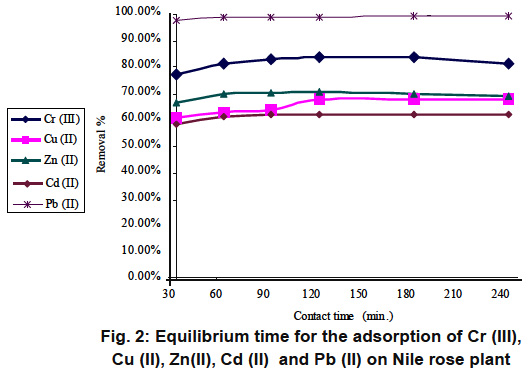 |
Figure 2: Equilibrium time for the adsorption of Cr (III), Cu (II), Zn(II), Cd (II) and Pb (II) on Nile rose plant Click here to view figure |
Adsorbents
Nile rose plant (water hyacinth) was collected from clean area far from those which may be industrially polluted. Rice husk was collected from agricultural areas. Both materials were then cutted into small pieces by using a clean cutter and oven dried at 80°C for 72 hours. The dried materials were grinded by using a clean electric mixer and then stored in clean plastic bags.
Chemicals
The aqueous solutions of Cu2+, Zn2+, Cd2+ and Pb2+ were prepared for this study by dilution of the stock 1000ppm standard solutions of the metals with deionized water to make their synthetic solutions. Solutions of 0.1 M NaOH and 0.1 M HNO3 were used for pH adjustment. Constant ionic strength 0.1N NaNO3 was used in all experiments. All chemicals used were of analytical reagent grade and were obtained from Merck, Germany.
| Figure 3: Effect of pH on the adsorption of Cr (III), Cu (II), Zn(II), Cd (II) and Pb (II) on rice husk Click here to view figure |
Adsorption Experiments
The batch studies were conducted by mixing each adsorbent with mixed Cu2+, Zn2+, Cd2+ and Pb2+ metallic solutions prepared in the laboratory. The samples were shaken at room temperature at 200 rpm, their content was filtered through 0.45 mm membrane filter (Whatman) using a vacuum pump, and the filtrate was, subsequently,analysed for its Cu2+ , Zn2+ , Cd2+ and Pb2+ contents using a ICP_OES Perkin Elmer optima 2000 DV inductively coupled plasma at wavelengths of 324.752nm,213.857 nm, 226.502nm and 220.353 nm for Cu (II), Zn(II), Cd (II) and Pb (II) metal ions, respectively.
Following the same procedure, different parameters were studied: contact time, pH, initial metal concentration and adsorbent dosage.
| Figure 4: Effect of pH on the adsorption of Cr (III), Cu (II), Zn(II), Cd (II) and Pb (II) on Nile rose plant Click here to view figure |
The solution–adsorbents mixtures were stirred at 200 rpm at varying time intervals (30,60,90,120,180,240) minutes at 25 ± 2 °C.
The effect of pH of the initial solution on the adsorption process was analysed using different solutions and adjusting the pH between 2.5 and 8.5 with NaOH and HNO3 to determine the optimum pH for maximum adsorption.
The effect of initial metal concentration was carried out with a constant 1g of each adsorbents using 50 mL solutions, containing varying metal ions concentration (5-50 mg/L) at 25 ± 2 °C. For each metal concentration, one sample was reserved for analysis to determine the initial metal concentration. The batch tests for the determination of the effect of initial metal concentration were conducted for the equilibrium time mixing at a constant speed of 200.rpm after adjusting the pH to the optimum value for maximum adsorption. Batch adsorption experiments at various adsorbents dosage (0.25,0.5,1.0, 1.5, 2 g) in 50 mL of solutions were also studied with the equilibrium time mixing at 200.rpm after adjusting the pH to the optimum value for maximum adsorption at 25 ± 2°C.
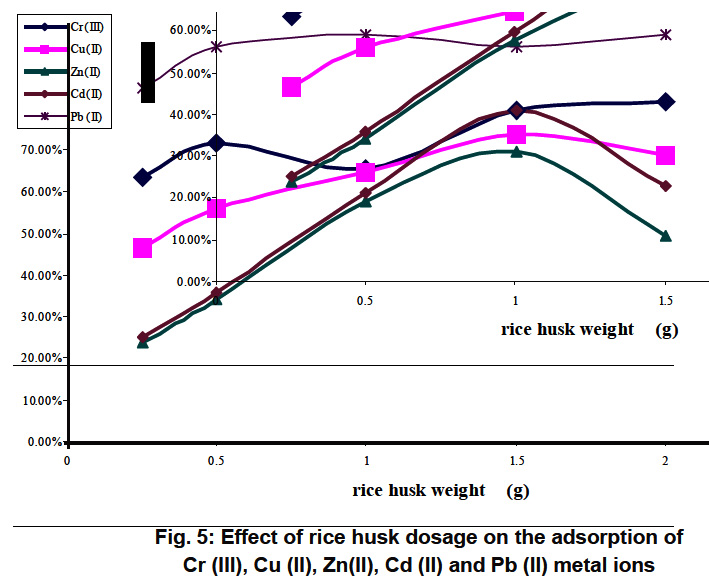 |
Figure 5: Effect of rice husk dosage on the adsorption of Cr (III), Cu (II), Zn(II), Cd (II) and Pb (II) metal ions Click here to view figure |
Case Study
A metal plant (COMEX industrial Co.) located at 6th of October city (an industrial city near Cairo) provided the rinsewater for bench scale studies by using the adsorbents under investigation. Samples were delivered from different rinsing tanks during one day shift to obtain close representation of the rinsewater as influenced by changes in daily operations. pH and heavy metal concentrations were determined. Wastewater samples were then subjected to treatment using the studied adsorbents.
Results and Discussion
Contact Time
Preliminary kinetic experiments were conducted to assess the time taken for the equilibrium to be attained and the results are presented in Fig. 1 for rice husk and Fig. 2 for Nile rose plant It is readily apparent from the figures that significant removals of all metals occurred in 90 min and no appreciable change in terms of removal was noticed after that. In all subsequent experiments,the equilibrium time was maintained at 90, which was considered as sufficient for the removals of Cr (III), Cu (II), Zn(II), Cd (II) and Pb (II) ions by either Rice husk or Nile rose plant.
 |
Figure 6: Effect of Nile rose plant dosage on the adsorption of Cr (III), Cu (II), Zn(II), Cd (II) and Pb (II) metal ions Click here to view figure |
It is also relevant to point out that, since active sorption sites in a system have a fixed number and each active site can adsorb only one ion in a monolayer,9 the metal uptake by the sorbent surface will be rapid initially, slowing down as the competition for decreasing availability of active sites intensifies by the metal ions remaining in solution. The rate of metal removal is of greatest significance for developing a natural adsorbent-based watertreatment technology.
Effect of pH on Adsorption
The pH-adsorption edges of the constant concentration of Cr (III), Cu (II), Zn(II), Cd (II) and Pb (II) for a constant adsorbent dose at 25 ± 2 °C are shown in Fig.3 for rice husk and Fig.4 for Nile rose plant. All experiments were carried out in the pH range of 2.5–8.5 where chemical precipitation is avoided, so that metal removal could be related to the adsorption process. As shown in Fig. 3 and 4, the maximum adsorption of Cr (III), Cu (II), Zn(II), Cd (II) and Pb (II) was found to occur at pH 4.5–6.5 on both of the investigated adsorbents. the adsorption of the studied metal cations increased as pH increases and recorded its minimum values at pH 2.5.This can be justified on the bases that at lower pH values, the H+ ions compete with the metal cation for the adsorption sites in the system, which in turn leads to partial releasing the later. The heavy metal cations are completely released under extreme acidic conditions.10
 |
Table 1: Values of Temkin constant for sorption of Cr (III), Cu (II), Zn(II), Cd (II) and Pb (II) ions Click here to view table |
The obtained results were also in agreement with the results of Asma Saeed et al.¹¹ for the adsorption of Pb (II), Cd (II), Cu (II), and Zn (II) onto a crop milling waste (black gram husk). They stated that sorption of all metals at pH 2 was negligible, and was then increasing with increase of pH.
This can be justified by the fact that: the availability of negatively charged groups at the biosorbent surface is necessary for the sorption of metals to proceed,10 which at the highly acidic pH 2 is unlikely as there is a net positive charge in the system due to H+ and H3O+. In such a system H+ compete with metal ions,12 resulting in active sites to become protonated to the virtual exclusion of metal binding on the biosorbent surface.2 This means that at higher H+ concentration, the adsorbent surface becomes more positively charged thus reducing the attraction between adsorbent and metal cations.15 In contrast, as the pH increases, more negatively charged surface becomes available thus facilitating greater metal uptake.16
Effect of Adsorbent Dosage on Adsorption
The effect of mass of adsorbent on the rate of uptake of Cr (III), Cu (II), Zn(II), Cd (II) and Pb (II) is depicted in Fig.5 for rice husk and Fig.6 for Nile rose plant. when the pH of the solutions was adjusted to the optimum pH range between 4.5 and 6.5. It is seen that the rate of removal of metal ions increased with the increase in the dose of adsorbent. It can be observed that removal efficiency of the adsorbent generally improved with increasing adsorbent dose up to a certain limit after which maximum adsorption sets in. This is expected due to the fact that the higher dose of adsorbents in the solution, the greater availability of exchangeable sites for the ions. It has to be mentioned also that he removal of Cd (II), Cu (II) and Zn (II) onto rice husk decreased as the dose of rice husk increased from 1.5g to 2g.This is in accordance with S.F. Montanher et al. 17 who found that the increasing of rice bran concentration resulted in a decreasing removal of the metal ions Cd (II), Cu (II), Pb (II) and Zn (II) per unit weight of adsorbent. This fact has been justified on the basis that some kind of hindrance, as a result of aggregation/agglomeration of sorbent particles at higher concentrations. Besides, the adsorption sites remain unsaturated during the sorption process due to a lower adsorptive capacity utilization of the sorbent. Therefore, a more economical for removal of a given amount of metal ions to be carried out using small several batches of sorbent rather than in a single batch.
 |
Table 2: Characterization of the rinse water from metal plating plant (COMEX) Click here to view table |
Adsorption Isotherm Studies
The analysis of equilibrium data for the adsorption of Cr (III),Cu (II), Zn(II), Cd (II) and Pb (II) on rice husk and Nile rose plant has been done by the Langumuir, Freundlich and the Temkin isotherm model. the data best fitted the Temkin isotherm given by the following equation :
Temkin equation. Where,
C = Concentration of adsorbate in solution at equilibrium (mg/L).
X=Amount of metal adsorbed per unit weight of adsorbent (mg/g) a & b are constants related to adsorption capacity and intensity of adsorption.
As mentioned above, the isotherm constants at specific pH values were determined from the respective plots, and are presented in Table 1. Regression values (R2) presented in Table 1, indicate that the adsorption data for Cu (II), Zn(II), Cd (II) and Pb (II) removal fitted well the Temkin isotherm for both of the adsorbents.
The Temkin isotherm fitted the present data because it takes into account the occupation of the more energetic adsorption sites at first. For natural unmodified materials such as the studied ones it is highly probable that their adsorption sites are energetically non-equivalent.18
Case Study
In order to widen the applicability of the removal technique, the optimised method was applied for the removal of Copper (II) and zinc (II) fom electroplating wastewater.
Table 2 shows the results of analysis of rinsewater. These results revealed the presence of copper and zinc in quantities higher than the permissible limits of the World Bank Standard for Electroplating Effluent Discharge into surface water and also higher than the permissible limits of metals in water according to the Egyptian environmental law.19-20
To study the effectiveness of the adsorbents under investigation on the removal of copper and zinc from the wastewater the optimum conditions obtained from the bench scale study were applied. The removal efficiencies achieved were 54.91% and 84.46 % for the removal of copper and zinc using rice husk. Nile rose plant showed removal efficiencies 16.86 % and 73.44 % for the removal of copper and zinc, respectively The results obtained in the case study were close to those obtained from the batch experiment using synthetically prepared wastewater.
Conclusion
The results obtained in this study clearly demonstrated the potential use of rice husk and Nile rose plant for the removal of Cr (III), Cu (II), Zn(II), Cd (II) and Pb (II) from mixed metal ions aqueous solutions. The following conclusions can be drawn based on the investigation:
- The kinetic studies indicated that equilibrium in the adsorption of Cr (III),Cu (II), Zn(II), Cd (II) and Pb (II) on the rice husk and Nile rose plant was reached in 90 min.of contact between the rice husk and Nile rose plant and the aqueous solution.
- The optimum pH corresponding to the maximum adsorption was found to lie between 4.5 and 6.5.
- The extent of adsorption for metals increased along with an increase of rice husk and Nile rose plant dosage.
- The experimental data were best fitted by the Temkin isotherm
- Rice husk and Nile rose plant showed good removal efficiencies in treating the electroplating wastewater, so that they can be considered as cheap materials that can be used as neutralising agents in the treatment processes.
The results are quite useful in developing an appropriate technology for designing a wastewater treatment plant.the process is economically feasible and easy to carry out.
References
- Weng C.H. and Huang C.P., "Treatment of metal industrial wastewater by fly ash and cement fixation. J. Envir. Eng, ASCE, (1994) 120(6): 1470-1487.
- B. Bayat, Combined removal of zinc(II) and cadmium(II) from aqueous solutions by adsorption onto high-calcium Turkish fly ash, Water Air Soil Pollut. (2002) 136: 69-92.
- G. Gupta and N. Torres, Use of fly ash in reducing toxicity of and heavy metals in wastewater effluent, J. Hazard. Mater. (1998) 57: 243-248.
- Ramos R. L., Bernal J. L.A., Mendoza B. J., Fuentes R. L.and Guerrero C.R.M., "Adsorption of zinc(II) from an aqueous solution onto activated carbon." Journal of Hazardous Materials (2002) 90(1): 27-38.
- Harvey N.W. and Chantawong V.,"Adsorption of heavy metals by ballclay: their competition and selectivity." Journal of Tokyo University of information sciences (2001).
- Yu L. J., Shukla S. S., Dorris K. L., Shukla A. and Margrave J. L. "Adsorption of chromium from aqueous solutions by maple sawdust." Journal of Hazardous Materials., (2003) 100(1-3): 53-63.
- Abdel-Ghani, N.T., Hefny, M., El-Chaghaby, Gh.A.F., "Removal of lead from aqueous solution using low-cost abundantly available adsorbents." Int. J. Environ. Sci. Tech., (2007) 4(1): 67-73.
- N. T. Abdel-Ghani , R.M. El-Nashar and G. A. El-Chaghaby., "Removal of Cr (III) and Pb (II) from solution by adsorption onto Casuarina Glauca tree leaves." In press in the Electronic journal of environmental , agricultural and food chemistry, Spain (2007).
- Langmuir I., "The adsorption of gases on plane surfaces of glass, mica and platinum." J. Am. Chem. Soc. (1918) 40: 1361.
- Forstner U. and Wittman G.T.W., "Metal pollution in the aquatic environment." Springer Verlag, Berlin-Heidelberg, New York, (1981) 21.
- Saeed A., Iqbal M. and Akhtar M. W., Removal and recovery of lead(II) from single and multimetal (Cd, Cu, Ni, Zn) solutions by crop milling waste (black gram husk)” Journal of Hazardous Materials., (2005) 117(1): 65-73.
- Luef, E., Prey, T., Kubicek, C.P., "Zinc biosorption by fungal waste mycelia." Appl. Microbiol. Biotechnol. (1991) 34: 688-692.
- Low K.S., Lee C.K. and Lee K.P. "Sorption of copper by dye-treated oil-palm fibers." Bioresour. Technol. (1993) 44: 109.
- Aldor I., Fourest, E and Volesky, B., "Desorption of cadmium from algal biosorbent." Can. J. Chem. Eng. (1995) 73: 516-522.
- Saeed A., Iqbal M. and Akhtar M.W. "Application of agricultural and wood wastes for the sorption of heavy metals in contaminated aqueous medium. Pakistan J. Sci. Ind. Res. (2002) 45: 206.
- Chang J. S., Law R. and Chang C.C., "Biosorption of lead, copper and cadmium by biomass of Pseudomonas aeruginosa PU21" Water Res. (1997) 31: 1651.
- Montanher S.F., Oliveira E.A. and Rollemberg M.C. "Removal of metal ions from aqueous solutions by sorption onto rice bran." Journal of Hazardous Materials., (2005) 117(2-3): 207-211.
- Kolasniski K.W. "Surface Science" Wiley, Chister, UK (2001).
- World Bank. "Pollution Prevention and Abatement: Electroplating Industry." Draft Technical Background Document. Environment Department, Washington, D.C (1996).
- Law number 4 of Promulgating the environmental law and its executive regulation, Egypt (1994).






