Sorption potentials of waste tyre for some heavy metals in aqueous solution
P.E. Omuku1 , A.K. Asiagwu1 , P.A.C. Okoye1 , I.A. Orji 1 and S.C. Ilabor2 *
1
Department of Pure and Industrial Chemistry,
Nnamdi Azikiwe University,
Awka,
Nigeria
2
Department of Chemistry,
Federal College of Education (Technical),
Asaba,
Nigeria
DOI: http://dx.doi.org/10.12944/CWE.3.1.06
An investigation into the adsorption potential of activated and unactivated waste tyre powders for some heavy metals (Pb2+ and Cd2+) in their aqueous solution has been studied. The result indicated that unactivated waste tyre is a good non-conventional adsorbent for the removal of Cd2+ from aqueous solution. A total of 93.3% of cadmium, contents was removed. The unactivated waste type proved a good absorbent for the removal of Cd2+ 5g of 500µm activated tyre removed over 86.66% of Pb2+ from solution.
Copy the following to cite this article:
Omuku P.E, Asiagwu A.K, Okoye P.A.C, Orji I.A, Ilabor S.C. Sorption potentials of waste tyre for some heavy metals in aqueous solution. Curr World Environ 2008;3(1):39-44 DOI:http://dx.doi.org/10.12944/CWE.3.1.06
Copy the following to cite this URL:
Omuku P.E, Asiagwu A.K, Okoye P.A.C, Orji I.A, Ilabor S.C. Sorption potentials of waste tyre for some heavy metals in aqueous solution. Curr World Environ 2008;3(1):39-44 http://www.cwejournal.org?p=92/
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2008-03-26 |
|---|---|
| Accepted: | 2008-05-16 |
Introduction
The tremendous increase in the use of heavy metals over the past few decades has inevitably resulted in an increased influex of metallic substances in aquatic environment (Harrison and Laxen 1980). Heavy metal pollution in the aquatic environment is a major health problem. This is because metals are toxic and non-biodegradable (Corapciogie and Hana 1987). Despite the toxicological effects of Cadium, it has a wide range of uses. The most significant use of cadmium is in the Ni-Cd batteries which are rechargeable. The batteries exhibit high output, long life, low maintenance and high tolerance to physical and electrical stress (Hoale 1978). Other uses of cadmium includes, metal coatings, pigments, stabilizers for pVC, in allays and ectropleting. It can also be employed in photo cells, photometry for ultraviolet sun-ray, filaments for incandescent high, electrodes and in denistry as an amalgam. (Okumen and Esther and David 1999). Similarly, lead has wide range uses. It is used in storage batteries, allays, solder, ceramics and plastic. Applied in petrol refining, halogenation, extraction, sulphoneation, manufacture of pigments, insulation cables and wiring household, printing inks glass, textiles and so on (Robert and Rowland). It is also toxic. These metals find their way into the aquatic environment through wastewater discharge (Gurper et al 2003). Because they are non-biodegradable, they tend to accumulate in aquatic organisms. Feeding in such aquatic organisms as fish, crabs or using such contaminated water can lead to metal poisoning in man. Heavy metals pose health hazards, if their concentrations exists the allowable limits. Even when the limits are not exceeded, there is still the potentials of a long term poisoning, since they are known to accumulate within biological systems (Quick, Wase and Fuster 1998). The increasing awareness of the environmental consequences arising from heavy metal accumulation of aquatic environment has lead to the demand for the treatment of industrial waterwater before discharge into aquatic environment.
Conventional treatment processes for metal contaminated wastewater include ion-exchange, chemical precipitation, ultra-filteration, electrochemical deposition and carbon adsorption. These methods though effective are not economically feasible for small and medium scale industries, because of the relatively high costs (Cheremshoff P. and Elizabeth 1979), Lelvani, Hubner, Weston and Matadrich 1998). Hence the need to explore cost effective alternative methods of adsorbants for the treatment of metals contaminated wastewater. There are some readily available waste materials, that posses potentials of cheap adsorbents for the treatment of metal contaminated wastewater. A number of workers have investigated such materials like melon seed husks, maize cob, groundnut husk and oil-palm fibre (Okamen Onyekpa 1989; Okeimen and Okunodye 1989; Okamen Okwuadia Okafor 1991; and Rowl, Lee and Leen 1993). There is still the need to investigate the adsorptive potentials of other cheap and readily available materials that may constitute the class of waste products in the environment. This present work is aimed at examining the potentials of activated and unactivated waste tyre in the removal of (adsorbent) of cadmium and lead from their aqueous solution.
 |
Table 1: Variation in adsorptivity of 2g waste tyre powder at different particle sizes Click here to view table |
Material and Methods
Waste tyre used in this work was a Michelin brand, collected from a vulcanizer in Onitsha, Nigeria. The tyre was sliced into bits, the metal-threading removal, while the sliced tyres was washed thoroughly with detergent and rinsed severally with clean water to remove any residue. The washed tyre was allowed to dry under the sun.
The dry waste tyre was then ground to powder using a crushing machine. The powder was sieved using the electrical vibration shaker to obtain particle sizes of 500µm, 250µm and 125µm.
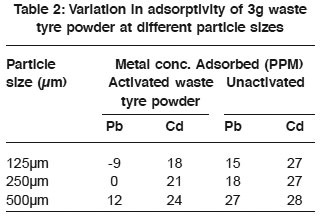 |
Table 2: Variation in adsorptivity of 3g waste tyre powder at different particle sizes Click here to view table |
The resulting waste tyre powder were subjected to chemical activation. Using hdrocloric acid. The activation process was carried out by mixing a known weight (1g, 2g, 3g & 5g) of waste tyre powder with a known volume of 1mHcl as the activating agent in a ratio of 20cm3 of 1MHCL to 250g of waste powder / Baks, Soumitra and Mahaja 1999), in an evaporating dish. The sample was allowed to dry at room temperature. Various masses of 1g, 2g, 3g and 5g for each of the three particle sizes were weighed and kept in a polyethylene away from moisture.
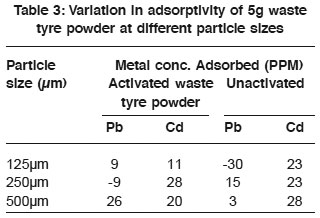 |
Table 3: Variation in adsorptivity of 5g waste tyre powder at different particle sizes Click here to view table |
The varying particle sizes of 500µm, 250µm and 123µm for activated and unactivated waste tyre powder were weighed out in 1g, 2g, 3g and 5g samples and were packed properly into different burettes and exactly 10mls of 20mg/l of standard Pb (NO3)2 and allowed for a contact period of 30m minus, for adsorption to take place. The burette tap was then opened after 30 minus and the solution was allowed to drain slowly and the eluent collected and labeled properly. This procedure was repeated for Cd (NO3)2. 4H2O standard solution (30mg/L). The eluent and the stock solution were assayed for metal ions (Pb2+ and Cd2+) left in the solution using the Atomic Absorption Spectrophotometer with air acetylene flame of 26000C at a measurement time of 4 seconds.
|
Metal |
flame tyre |
lamp wavelength |
|
Cd |
Air Acetylene |
228.8nm |
|
Rd |
Air acetylene |
217.0nm |

Results and Discussion
The findings of this work are contained in Tables 1 to 7. Table 1, show the variation of 1g waste tyre powder at different particle sizes. The data below showed that the uanctivated waste tyre, had very high adsorptivity as compared to that of the activated one. This is an indication that the activation did not really increase the porosity and internal surface area of the adsorbent. The adsorpitivity of Cd2+ as compared to that of Pb2+ were high. The waste tyre whether activated or not functions as good adsorbent for the adsorption of Cd2+ from aqueous solution. However, it is worth noting that activated tyre proved a poor adsorbent for the removal of lead ion from solution via 1g mass of adsorbent. The particle size, did not have any appreciable influence on the adsorption of Cd2+ and Pb2+, but there was noticeable difference in the adsorptivity of activated waste tyre powder of Pb2+. This observation is at variance with the theory which states that absorptivity increase with increase in particle size. (Vasenth Kumer, Subanandam Ramamurthi and Sivanesan 2004).
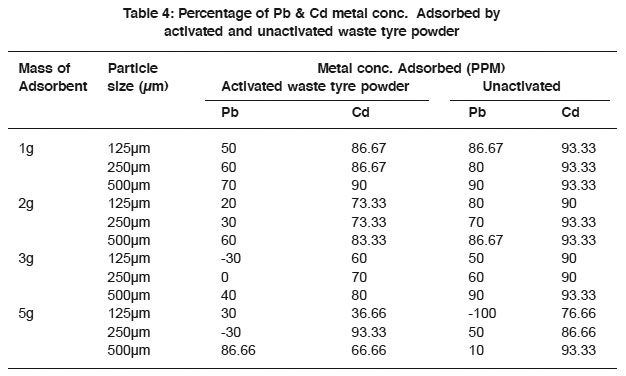 |
Table 4: Percentage of Pb & Cd metal conc. Adsorbed by activated and unactivated waste tyre powder Click here to view table |
Table 2 show that the unactivated waste tyre is a better absorbent for the removal of Pb2+ and CD2+ acid 2+ from aqueous solution as compared to the activated waste tyre powder. The unactivated waste tyre had higher adsoptivity, though Cd2+ is more favoured. However, the activated waste tyre did not show the properties of a good absorbent in respect to the adsorption of Pb2+ from aqueous solution. Activation did not increase the adsorption of Pb2+ ion, but increase in particle size, showed increased adsorption.
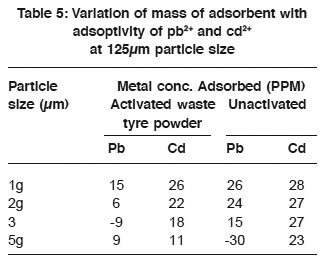 |
Table 5: Variation of mass of adsorbent with adsoptivity of pb2+ and cd2+ at 125μm particle size Click here to view table |
The variation in adsorption potential of 3g waste tyre powder at varying particle sizes is shown in table 3. The Cd2+ showed high affinity for waste tyre powder. It was observed that activation did not increase adsorptivity as the unactivated adsorbent has high adsorption ability than the activated. Also the adsorption of Pb2+ by activated waste tyre showed great deviation as there was increase in metal concentration in one of the samples than in the stock solution. Using an adsorbent of 5g (Table 4). It was observed that unactivated waste tyre powder, has high adsorptivity than the activated one. Similarly, adsorptivity increase gradually with increase in particle size, which theoretically had to be the reverse since smaller particle size has large internal surface area available as adsorption site (Ford 1981). This deviation may be due to the fact that smaller particle size floated in the column when the adsorbent was added. Similarly it was observed that the activated waste tyre showed improved adsorption with increase in mass of the adsorbent. However, Pb2+ was greatly adsorbed by 500mm of 5g absorbent as shown in table 4.
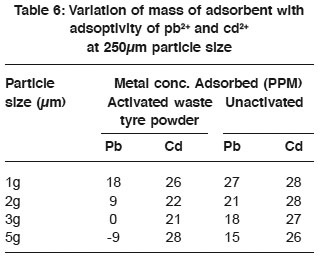 |
Table 6: Variation of mass of adsorbent with adsoptivity of pb2+ and cd2+ at 250μm particle size Click here to view table |
Table 5, represent percentage of pb and Cd metal concentration adsorbed by activated and unactivated waste powder. The results showed that the percentage of Cd2+ adsorbed was high compared to Pb2+ and that the unactivated waste tyre proved a good adsorbent in the adsorption of Cd2+ from solution. However, the activated waste tyre also appreciably adsorbs Cd2+. It was generally observed that the increase in mass of the adsorbent did not greatly influence the adsorption capacity, but increase in mass had significant effect in the adsorption of lead was, where it had the maximum adsorption with activated waste tyre powder 2+ 5g mass adsorbent and 500µm particle size.
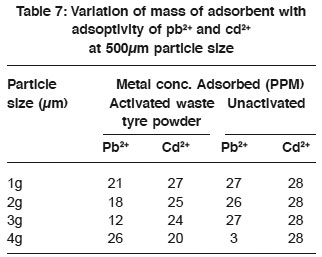 |
Table 7: Variation of mass of adsorbent with adsoptivity of pb2+ and cd2+ at 500μm particle size Click here to view table |
Variation of mass of adsorbent with adsorptivity of Pb2+ and Cd2+ at 125mm particle size Table 6 showed that the adsorptivity of metal ions from solution decreased with increase in the mass of adsorbent. Also the general trend of abdsorptivity (Krishnan and Concilia 1987) decrased with increase in mass attributed to the quantity of the adsorbent floating on top of the adsorbent, being much, but only a little surface area of the adsorbent is in contact with adsorbate. 250µm of 5g activated waste powder was observed to be a good adsorbent for Cd2+ as the adsorptivity increased appreciably.
For 500µm particle size the variation of mass of adsorbent with adsorptivity of metal ions (Pb2+ and Cd2+) (Table 7) showed that the variation in mass did not effect the adsorptivity of Cd2+ by unactivated waste tyre of 500µm particle. This was evident because metal concentration adsorbed remained the same despite change in mass (Azab and Peterson 1989). The metal concentration adsorbed using activated waste tyre showed that the adsorptivity of Cd2+ decreased with increase in mass of absorbent, while that of Pb2+ had maximum adsorption with increase in mass.
Conclusions
The activated and the unactivated waste tyre powder are good adsorbent for the removal of Cd2+ from aqueous solution. However, the unactivated waste tyre powder proved a better adsorbent as such, resources should to be spent on activation of the waste tyre powders, since it did not actually increased the porosity of the adsorbent. The particle size of 500µm proved the ideal particle size of waste tyre powders that gave appreciable results in the adsorption of Cd2+ from aqueous solution.
Variation in the mass of the adsorbent did not really affect the adsorptivity, as such any suitable mass within the range of that used in this research can be used practically. However, unactivated waste tyre powder showed over 90% maximum efficiency for the removal of Pb2+ from aqueious solution. While the activated one proved good adsorbent only when 5g mass adsorbent was used with 500µm particle size. Therefore the unactivated waste tyre powder can be practically utilized in the removal/adsorption of Pb2+ and Cd2+ from actually polluted waste water from industries.
References
2. Cheremshoff, P. and Elizabeth F., Carbon Adsorption. Handbook, Michigan Am Arbor Science Series (1979) 15: 1-2.
3. Corepciogie M. O. and Henry C. P., The adsorption of heavy metals onto hydrous activated carbon; Water resources (1987) 21(9): 1031-1044.
4. Ford, D. L., Waste water Charcteristics and treatment, Activated carbon adsorption for waste water treatment CRC Press Inc. (1981)1-28.
5. Gurper W. K., Jaln C. K. Ali J, Sharma M and Sani V. K., Removal of Cadmium and Nickel from wastewater using biagasse fly ashsugar industry waste. Water Resources (2003) 37: 4038-4044.
6. Harrison R. M. and Laxen D. P. H., Metals in Environmental Chemistry Chem, Br. (1980) 16: 316-320.
7. Hoale, R.A., The Chemistry of Environment. John Willey and Sons. New York. (1978) 15-20.
8. Krishnan, S. S., Concilla A. and Jarvis P. E., Industrial waste treatment for toxic heavy metals, using natural materials as adsorbent, Radiology and Nuclear Chem., (1987) 110: 373-378.
9. Lelvan, S. B. Hubner, A. Weston A. and Matadich N., Removal of Hexavalent and metal cations by selective and novel carbon adsorbent, Carbon (1998) 36: 219-226.
10. Low, K. S. Lee C. K. and Lee K.P., Sorption of Copper by treated oil palm fibre. Bioresources Tech. (1995) 14: 109:112.
11. Okeimen, F. E. and Okunodye, J. N., Removal of Cadmium and Copper Ions from aqueous solution with maize cob. Biological waste. (1989) 30: 325-330.
12. Okamen F. E. and Onyekpa V.U., Removal of heavy metal ions from aqueous solution with melon seed husks. Biological waste. (1989) 29: 11-16.
13. Okeimen F. E. Esther U. O. and David E.O., Sorption of Cadium and Lead ions on modified groundnut husk. Nigeria-Chem. Tech. Biotechnology., (1999) 31: 97-99.
14. Qunck S.Y,., Wase D. A. and Foster C.F., Water, S.A. (1998) 24(3): 251-255.
15. Roberts E.J. & Rowland S.P., Environmental Science and Tech. (1973) 7: 552-555.
16. Vasanth Kummer K, Subanandam V. Ramamurthi S. and Sivanesan S., Solidliquid adsorption for waste water treatment, principle, design and operation (2004).






