Heavy metal status and physicochemical properties of agricultural soil amended by short term applicaton of animal manure
S.A. Odoemelam1 * and O. Ajunwa1
1
Department of Chemistry,
Michael Okpara University of Agriculture,
Umudike, P. M. B. 7267,
Umuahia,
Abia State
Nigeria
DOI: http://dx.doi.org/10.12944/CWE.3.1.03
A survey was conducted to estimate short term heavy metal build up in arable soils amended with animal manure. Soil samples were collected from parts of the Michael Okpara University of Agriculture farm amended with animal manure and analyzed for heavy metal concentration and physicochemical properties. Zinc was the most abundant mineral with a mean concentration of 112.34 mg/kg, while Cd had the lowest mean concentration of 2.16 mg/kg. Lead and Chromium had the mean concentrations of 14.90 and 16.96 mg/kg, respectively. Arsenic was, however, not detected. Calcium was the most abundant exchangeable base followed by Mg, K and Na. Exchangeable acidity ranged from 1.60 to 2.40 while available P ranged from 5.40 to 67.81. Physicochemical properties of the samples showed that the soils were loamy with 77-91 % sand, 6.00 -11.9 % clay and 2.89-13.91 % silt. Soil pH in water ranged from 5.16 to 7.19. The application of animal manure affected physicochemical properties and caused heavy metal enrichment of the agricultural soils studied.
Copy the following to cite this article:
Odoemelam S.A, Ajunwa O. Heavy metal status and physicochemical properties of agricultural soil amended by short term applicaton of animal manure. Curr World Environ 2008;3(1):21-26 DOI:http://dx.doi.org/10.12944/CWE.3.1.03
Copy the following to cite this URL:
Odoemelam S.A, Ajunwa O. Heavy metal status and physicochemical properties of agricultural soil amended by short term applicaton of animal manure. Curr World Environ 2008;3(1):21-26. Available from: http://www.cwejournal.org/?p=732
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2008-04-18 |
|---|---|
| Accepted: | 2008-06-02 |
Introduction
As a result of the high cost of inorganic fertilizers, small-scale farmers now apply animal manures, sewage sludge and municipal waste to improve soil fertility. Amendment of agricultural soil with domestic refuse and animal manure provides a cheap means of enriching the soil with plant nutrients and organic matter. However, this may lead to elevated levels of heavy metals in soils and possible risk of eutrophication. Though some of these heavy metals are useful to plants and animals when they are present at trace levels or in the required proportion, they may, however, be dangerous when they accumulate in large quantities (Tuzen, 2003). Kidd et al., (2007) have reported that metal behaviour in soils and plant uptake are dependent on the nature of the metal, soil physicochemical properties and plant species. It is pertinent that for soil to fulfill its function in agricultural production and as a habitat for numerous beneficial microorganisms, heavy metal accumulation has to be minimized to a level that is not deleterious to the agro-ecosystem. Thus long-term accumulation of heavy metals in agricultural soils may cause serious (ecological) problems if tolerance levels are exceeded.
The soil acts a long-term sink for heavy metals, some of which have long residence times, depending on the element and soil characteristics (Alloway, 1995). More often heavy metals input to agricultural soils may come from inorganic fertilizer application. Other major sources of heavy metal input to agricultural soils include atmospheric deposition, sewage sludge, agro-chemicals, livestock manures, irrigation water, industrial wastes and compost (Nicholson et al., 1999; Alloway, 1995; Zhou et al., 2005).
Waste-amended soils have been reported to have high organic matter content, which affects the degree of aggregation of soil particles, reduces bulk density and increases porosity of soils (Anikwe and Nwobodo, 2002). Continuous application of animal manures and municipal wastes on soils may increase heavy metal concentration in soils (Conway and Pretly, 1991; Anikwe and Nwobodo, 2002). Thus, large scale use of these secondary raw material fertilizers should be handled with caution.
The environmental safety of utilizing livestock and poultry manure for intensive farming is now attracting great attention because the manures often contain high concentration of heavy metals and organic pollutants. Kidd et al., (2007) reported that metals derived from waste biomass are generally organically bound and less available for plant uptake than the more mobile salt impurities found in commercial fertilizers. On the other hand, Conway and Pretly (1991) argue that soils on which poultry and pig manures are continuously applied at high rates accumulate heavy metals, thus jeopardizing the good functioning of soil, contaminating crops and posing human health risks. In the same vein, Cang et al., (2004) have suggested that the application of organic fertilizers largely derived from bio-wastes should be strongly regulated to forestall unwarranted heavy metal enrichment of arable soils. The monitoring of heavy metals in the agro-ecosystem is very important since metals do not only bioaccumulate but also biomagnify from one trophic level to the other. The aim of this study is to assess the short-term effect of arable soil amendment with animal manure on soil physicochemical properties and heavy metal content.
Material and Methods
Study Area
The study area is located within Michael Okpara University of Agriculture, Umudike, Nigeria. It is part of the University’s farmland where animal manure was applied to improve soil fertility. Umudike is about 10 km south-east of Umuahia, capital of Abia State of Nigeria and is located between latitudes 05° and 05° 25'N and longitudes 07o and 07 05'E.
Sample Collection
The sampling area was divided into five stations. Three soil samples were randomly collected from three sampling sites in each station and pooled together to obtain a composite sample. Soil samples were taken in depths of approximately 0-15 cm and 15 -30 cm using a soil auger. The soils were air-dried, ground and screened through a 2 mm sieve and transferred to polythene bottles until analysis.
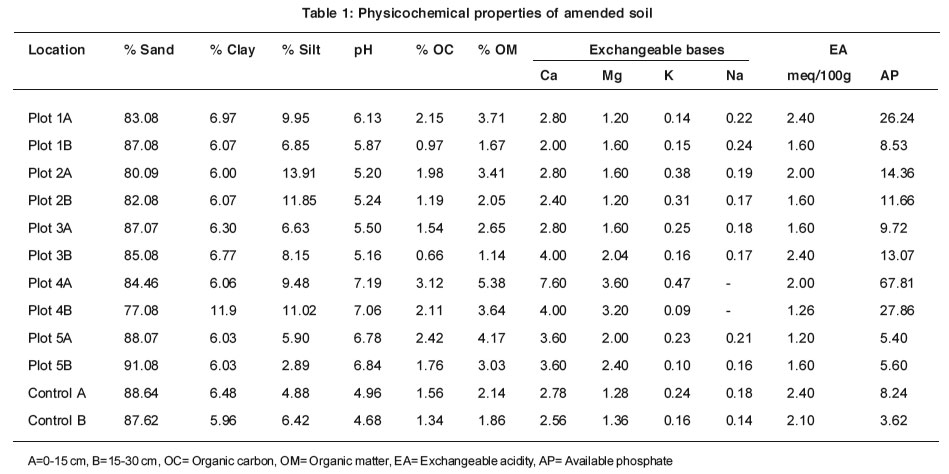 |
Table 1: Physicochemical properties of amended soil Click here to view table |
Soil Analysis
All determinations were carried out in triplicate on representative soil samples (< 2 mm fraction). Samples of each soil were analyzed for particle size (sand, silt and clay) by hydrometer method (Bouyoucos, 1951; 1962). Soil pH was measured in H2O using a 1:1 soil: water ratio (Black, 1965). Organic matter was analyzed by the dichromate oxidation method (Walkley and Black, 1934). Exchangeable cations were extracted with 1 M ammonium acetate (Jackson, 1958). Potassium and sodium in the extracts were determined by flame photometry while Ca and Mg were determined by atomic absorption spectrophotometry (AAS, Bulk Scientific Atomic Absorption/Emission Spectrophotometer 200 A). Available P was determined colorimetrically using the molybdenum blue method (Murphy ands Riley, 1962). Exchangeable acidity was estimated by titration as described by McLean (1965).
For heavy metal analysis soil samples (0.5 g) were placed in 100 ml beakers and digested with 10 ml of concentrated HCl: HNO3 (3: 1) mixture. The residue was cooled, diluted to 30 ml with deionized water and filtered. Heavy metal concentrations in the prepared samples were determined by atomic absorption spectrophotometry.
Results and Discussion
Soil Physicochemical Properties
Some selected physicochemical properties of the soil samples are presented in Table 1. The soils are predominantly sandy loam with clay and silt contents ranging from 6.00 to 11.9% and 2.89 to 13.91%, respectively. The results indicate that the sand fraction of the soil decreased slightly with soil depth in Plots 1, 2 and 5 whereas the reverse was the case in Plots 3 and 4. The sandy nature of the soils makes amendment with animal manure suitable because the high permeability of the soil will allow large quantities of leachates to pass through the soil thereby making crops to absorb nutrients readily.
Organic carbon (OC) and organic matter (OM) contents were higher in the upper layer in all the plots. The higher levels of OM present in the upper layer of all the plots could be attributed to the application of animal manure. An increase in the OM content of soil was found after amendment of agricultural soils with sewage sludge (de las Heras et al., 2005). Kidd et al., (2007) and Anikwe and Nwobodo (2002) also reported that long term application of biosolids on agricultural soils increased soil OM tremendously. Enweozor et al., (1988) classified soil OM content of < 2.0 % as low; 2.1- 3.0 % as medium and > 3.1 as high. Following this classification the amended agricultural soil investigated had high OM in the upper layers except Plot 3. Organic matter acts as a reservoir of both essential and non-essential mineral elements for plant growth and development. Thus increased OM content may lead to increased soil fertility. High OM content tends to buffer the soil by preventing excessive pH changes due to the release of exchangeable cations during mineralization of OM (Anikwe and Nwobodo, 2002).
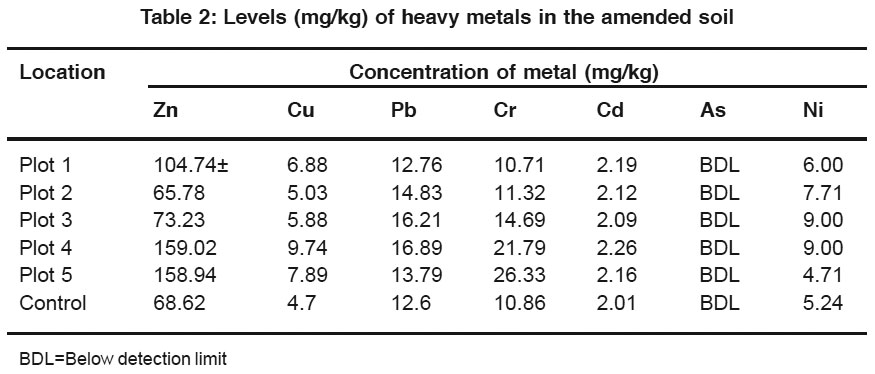 |
Table 2: Levels (mg/kg) of heavy metals in the amended soil Click here to view table |
The soil pH in water ranged from 5.16 to 7.19. Soil pH was higher in the amended soil than in the control soil sample (Table 1). Animal manure application increased soil pH and therefore led to a decrease in exchangeable acidity (Table 1). Exchangeable acidity was decreased from average value of 2.25 to 1.77 by the application of animal manure. Several studies have shown that the application of biosolids such as animal manure and compost on acid soils increases the soil pH appreciably (Hue and Amien, 1989; Anikwe and Nwobodo, 2002; Ano and Ubochi, 2007; Kidd et al., 2007). According to Isirimah et al., (2003) most plants and soil microorganisms thrive best in soils of pH between 6 and 7.5. The increased pH in the animal manure-amended soil may be a positive productivity indicator in an acidic tropical soil where low pH limits uptake of nutrients (Anikwe and Nwobodo, 2002).
There were remarkable increases in soil organic matter, exchangeable bases and available phosphorus following short-term land application of animal manure. It is therefore believed that these parameters will increase tremendously if animal manure is applied for a long time.
Heavy Metal Concentration
The concentrations of heavy metals were higher in the amended soils compared to the unamended (control) soil (Table 2). Zinc was the most abundant metal determined with a mean concentration of 112.34 ± 45.02 mgt/kg (range: 65.78 to 159.02 mg/kg). Plots 4 and 5 had the highest levels of Zn followed by Plot 1 while Plot 2 ranked last in Zn concentration. Zinc is known to be added to animal feed as a nutritional supplement. Perhaps this may account for the high level of Zn recorded in this study.
The concentration of Cu ranged from 5.03 to 9.74 mg/kg with a mean value of 7.08 mg/kg. Chromium ranked second in order or abundance (mean = 16.96 mg/kg). The highest concentration was recorded for Plot 5 (26.33 mg/kg) while the lowest concentration of 10.71 mg/kg was recorded for Plot 1. Cadmium was the least abundant element in the soil samples studied with a mean concentration of 2.16 mg/kg. Cadmium is a nutritionally undesirable and toxic element, which is not added to animal feed. Therefore it cannot be said to have been transferred from feed to animal manure and then to soil. The concentration of Ni ranged from 4.71 to 9.00 mg/kg. Nickel is another element that is not normally desired in animal feed. Plots 3 and 4 had the highest Ni level of 9.00 mg/ kg. Lead was the 3rd most abundant metal in the soil samples studied with a mean concentration 14.9 mg/kg. Soil lead concentration was highest in Plot 4 followed by Plot 3 while Plot1 had the lowest concentration of 12.76 mg/kg.
All the metals had positive correlation (r ranged from 0.0232 to 0.9896) with one another except with Ni (Table 3). However, Pb was the only metal that positively correlated with Ni (0.8752) as well as with the other metals. The correlation coefficient shows the level of interaction between the metals in the soil.
References
- Alloway, B. J., Soil processes and the behaviour of metals. Kluwer Academic Publishers, Dordrecht. (1995) 11-37.
- Anikwe, M. A. N. and Nwobodo, K. C. A., Long term effect of municipal waste disposal on soil properties and productivity of sites used for urban agriculture in Abakaliki, Nigeria. Bioresource Technol. (2002) 83: 241-250.
- Ano, A. O. Ubochi, C. I., Neutralization of soil acidity by animal manure: mechanism of reaction. Afr. J. Biotechnol. (2007) 6(4): 364-368.
- Black, C. A., Methods of soil analysis. Agronomy No 9. Part 2. American Society of Agronomy. Madison, Wisconsin (1965).
- Bouyoucos, G. J., Direction for mechanical analysis of soils by hydrometer method. Soil Sci. (1951) 42: 225-229.
- Bouyoucos, G. J., Hydrometer method improved for making particle size analysis o soils. Agron. J. (1962) 54: 464-465.
- Cang, L., Wang, Y. J., Zhou, D. M. and Dong, Y. H. Heavy metal pollution in poultry and livestock feeds and manures under intensive farming in Jiangsu Province, China. J. Environ. Sci. (China), (2004) 16(3): 371-374.
- Conway, G. R. and Pretly, J. N., Unwanted harvest, agriculture and pollution. Earthscan Publication, London (1991).
- De Las Heras, J., Maòas, P. and Labrdor, J., Effects of several applications of digested sewage sludge on soils and plants. J. Environ. Sci. Heal. A (2005) 40: 437-451.
- Enweozor, W. O., Ohiri, A. C., Opubaribo, E. E., Udoh, E. J., A review of soil fertility investigations in southeastern Nigeria. HFDA, Lagos, Nigeria (1988).
- Hue, N. V. and Amien, I., Aluminium detoxification with green manures. Comm. Soil Sci. Plant Anal. (1989) 20: 1499-1511.
- Jackson, M. L., Soil chemical analysis. Prentice Hall, NY (1958).
- Kidd, P. S., Domínguez-Rodríguez, M. J., Diez, J. and Monterroso, C., Bioavailability and plant accumulation of heavy metals and phosphorus in agricultural soils amended by long-term application of sewage sludge. Chemosphere. (2007) 66: 1458-1467.
- McLean, E. O., Aluminum. In: Methods of Soil analysis. Agronomy No 9. Part 2. American Society of Agronomy. Madison, Wisconsin (1965).
- Murphy, J. and Riley, J. P., A modified single solution method for the determination of phosphate in natural water. Anal. Chim. Acta (1962) 27: 31-36.
- Nicholson, F. A., Chambers, B. I., Williams, J. R. and Unwin, R. J., Heavy metal content of livestock feeds and animal manures in England and Wales. Bioresource Technol. (1999) 70: 23-31.
- Tûzen, M., Determination of heavy metals in soil, mushroom and plant samples by atomic absorption spectrometry. Microchemical Journal. (2003) 74: 287-297.
- Walkley, A and Black, I. A., An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. (1934) 37: 29-38.
- Zhou, D. M., Had, X. Z., Wang, Y. J., Dong, Y. H. and Cang, L., Copper and zinc uptake by radish and pakchoi as affected by application of livestock and poultry manures. Chemosphere. (2005) 52(3): 167-175.






