Determination of water quality of different areas of Kaithal,Haryana
Anurag Khatkar1 * and Neha Garg2
1
R.K.S.D. College of Pharmacy,
Kaithal,
India
2
Zublliant Pharmaceuticals,
Noida,
India
DOI: http://dx.doi.org/10.12944/CWE.3.1.17
Ground water which were taken from the various places of in and around Kaithal town were analyzed and the analysis reports that the water quality parameters like pH, EC, Cl-,TDS ,Ca2+, Mg2+ and Hardness lies within the maximum permissible limit prescribed by WHO and ICMR. Except few parameters like DO, few samples were reported with lower DO than the permissible level, but this value does not have any impact for the water to use for drinking purpose.
Copy the following to cite this article:
Khatkar A, Garg N. Determination of water quality of different areas of Kaithal,Haryana. Curr World Environ 2008;3(1):123-126 DOI:http://dx.doi.org/10.12944/CWE.3.1.17
Copy the following to cite this URL:
Khatkar A, Garg N. Determination of water quality of different areas of Kaithal,Haryana. Curr World Environ 2008;3(1):123-126. Available from: http://www.cwejournal.org/?p=779
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2008-03-30 |
|---|---|
| Accepted: | 2008-05-06 |
Introduction
Water is the most fundamental element for life, as survival is impossible without it. Mostly the ground water contains different kinds of chemical constituents. The kinds and amount of constituents depend upon the geo-chemical environment, movement and sources of water. The concentration of dissolved constituents in groundwater is comparatively higher than surface water. The quality of ground water depends on various chemical constituents and their concentration, which is mostly derived from the geological data of the particular region.
Panda and Singh (1996) studied the major inorganic anions such as Nitrate, Sulphate, Fluoride, Chloride, and Phosphate in drinking water collected from 5 different sources in Port City Paradeep and the results showed seasonal fluctuations. Naidu et al. (1998) studied the water quality parameters in 3 north coastal town of Andhra Pradesh and the results indicate that, the water of Mindi Industrial Zone, Old Post Office and Jalaripet of Visakhapatnam, Kothapeta, Vakumpeta and Mayuri, theatre areas of Vizianagaram and Kothapeta and complex areas of Srikakulam towns are polluted either by industrials waste water or by sewage and saline waters. Kaplay et al. (1998) studied the quality of Bore Well and Dug Well water around the industrial areas of Tuppa region of Nanded City, Maharashtra and the study reveal that, the ground water is contaminated due to industrial effluent and Total Hardness, Salinity, Calcium and Magnesium content as per Indian standards for drinking water. In our study, a total of eight water samples from hand pumps, tube wells and Govt. supply used by people of Kaithal were collected in clean polythene bottles brought to the laboratory. The samples were chemically preserved by the addition of 3-5 ml concentrated HNO3 per litre of the sample.
Material and Methods
All the chemicals used were LR grade and some were AR grade, procured from various chemical units like Merck, Mumbai; Qualigens, Mumbai and s.d.Fine, Mumbai. . The temperature, pH, conductivity and dissolved solids of the water samples were determined on the spot using a thermometer; pH meter, conductometer and TDS meter. Various standard methods (APHA-AWWA-WPCF, 1995; HMSO, 1986)1 were used for the determination of other parameters. Total alkalinity was determined by visual titration method using methyl orange and phenolphthalein as indicator. Total hardness and calcium were measured by EDTA titrimetric method using EBT indicator respectively. Chloride was determined by Argentometric method using potassium chromate indicator.
Results and Discussions
The physiochemical properties of water samples from various sites of Kaithal city were presented in Tables. The data revealed that there were considerable variations in the examined samples from different sources with respect to their chemical characteristics. The results indicate that the quality of water considerably varies from location to location. The underground water is characterized by a relatively constant pH of around 6.0 and 6.50 for the samples collected from Friends Colony and Rishi Nagar. Water samples with low pH may be attributed to the discharge of acidic water into these sources by the agricultural and domestic activities. Samples collected form the Sampling points Park Disposal Area(8.23) and Anaj Mandi Area(7.38) were slightly basic which can be seen from its pH and alkalinity values. In fact 98% of all world ground water are dominated by Calcium and bicarbonate ions due to lime stone weathering in the catchments and under ground water beds2. In water buffered by the presence of bicarbonate, carbonate and hydroxyl ions, this temperature effect is modified. Though pH has no direct effect on the human health, all the biochemical reactions are sensitive to variation of pH. For most reaction as well as for human beings, pH value 7.0 is considered as best and ideal. In the present study pH value of water samples varied in a narrow range within the permissible limits in all sources. The pH is of the utmost importance in determining the corrositivity of water. In general, the lower the value of pH, the higher the level of corrosion. It has been observed that in some cases decrease in pH is accompanied by the increase in bicarbonate, carbonate and hydroxyl ions. Decrease in pH can be caused by the increase in the amount of organic carbon, total carbonate by the use of sewage.
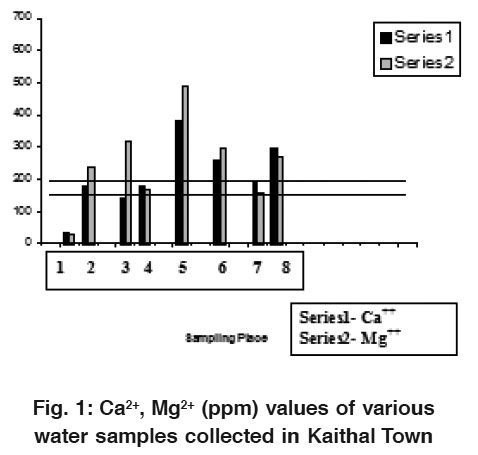 |
Figure 1: Ca2+, Mg2+ (ppm) values of various water samples collected in Kaithal Town Click here to view figure |
pH was positively correlated with electrical conductance and total alkalinity. The EC values were found higher at sampling points Friends Colony and Park disposle Area due to concentrated colloids in canal water and dissolved salts in Anaj Mandi. Very low conductivity was found at sampling points Rishi Nagar, Ram Nagar & Hanuman Mandir Area. The values of salts such as Ca, Mg, Cl- and SO42- suggested that the surface water sources posses’ very low components, while underground water sources which are far away from surface ground water or Dam water source were found to possess higher amounts of components. Conductance is a function of water, hence a standard temperature, usually 250C, is specified in reporting conductivity. Higher the concentration of electrolytes in water is its electrical conductivity. Conductance is a function of water, hence a standard temperature, usually 250C, is specified in reporting conductivity. Higher the concentration of electrolytes in water the more is its electrical conductance.
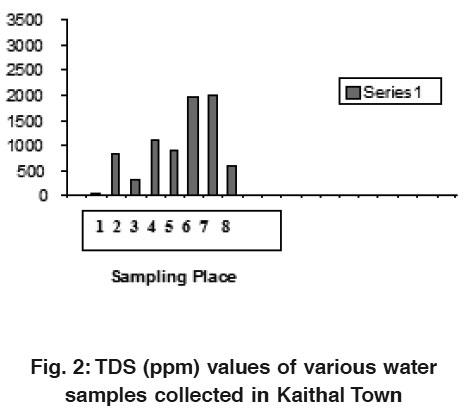 |
Figure 2: TDS (ppm) values of various water samples collected in Kaithal Town Click here to view figure |
In the present study, electrical conductance was highest in water sample of Friends Colony and Park disposle Area. It is well known that the conductance of water increases with salts. Total dissolved solids and conductivity can be used to delineate each other. Conductivity is proportional to the dissolved solids. Both showed analogous trend in seasonal variation (Patel, et al, 1994).3
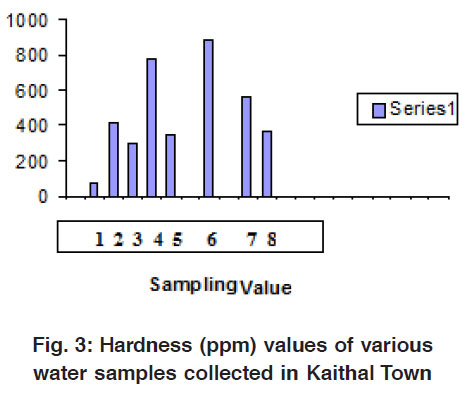 |
Figure 3: Hardness (ppm) values of various water samples collected in Kaithal Town Click here to view figure |
The water samples from Anaj Mandi area was found to possess high TDS value when compared with the tolerance limit of 1500 ppm. The TDS was found to be low for the water samples collected from the Friends Colony and Rishi Nagar. Samples from the sources Public Club Area, Hospital Area and water Works area showed low level of TDS of the range less than 250 ppm, which indicates that the recharging of under ground water through either rain water or by the water from near by canals. The possibilities of dissolution of rockey minerals are very low. TDS in all the samples found nil which is a good indicator that there is no direct seepage of contaminated surface water or any sewage water into the water sources. The Dissolved Oxygen of the water samples varies from 4.0 to 8.8 ppm from the all sampling locations.
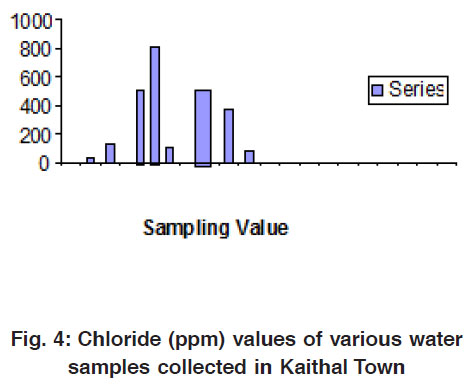 |
Figure 4: Chloride (ppm) values of various water samples collected in Kaithal Town Click here to view figure |
Alkalinity in terms of HCO3- of all these water samples ranged from 0.0- 1930 mg/L respectively. Chloride contents of these water samples ranged from 35-875 mg/L respectively. Calcium of water samples ranged from 25- 425 mg/ L, respectively. Magnesium content of all water samples ranged from 35-485 mg/L. Total alkalinity is a measure of the ability of the water to neutralize acids. The constituents of alkalinity in neutral system include mainly carbonate, bicarbonate, hydroxide and other components which may contribute to alkalinity are H2BO32-, HPO42-, HS- and NH3O. These compounds result from dissolution mineral substances in the soil and atmosphere (Mittal and Verma, 1997).4
Alkalinity is a big problem for industries also, as alkaline water if used in boilers for steam generation may lead to precipitation of sludge, deposition of scales and cause caustic embrittlement. This study also indicates that any industry establishment in this area must have alkalinity treatment plant prior to use of ground water or should go for some alternate water source.
Water hardness is the traditional measure of the capacity of water to react with soap, hard water requiring considerably more soap to produce lather. Hardness is one of the very important properties of ground water from utility point of view for different purposes. In the present study water was very hard and crossed the permissible limits. It is well known that hardness is not caused by a single substance but by a variety of dissolved polyvalent metallic ions, predominantly calcium and magnesium cation, although other cation likes barium, iron, manganese, strontium and zinc also contribute. The high concentration of total hardness in water samples may be due to dissolution of polyvalent metallic ions from sedimentary rocks, seepage and run off from soil. As we know calcium and magnesium, are the two principal ions. The concentration of total hardness in drinking water sources ranged between 75 and 1110 mg/L (Nawlakhe; 1995)5, (Sastry et al )6 also reported water samples from ponds, wells and hand pumps were very hard ranging from 222.8-1094.4 mg/L. In Anaj Mandi Area tube well and well water showed high concentration of total hardness.
In the present study total hardness was positively correlated with fluoride, chloride, calcium and magnesium. The strong correlation-ship between these parameters could be due to changes in land use namely deforestation, disruption in internal sources of hardness and alkalinity, climatic factor or industrialization.
As calcium and magnesium are directly related to hardness and hence combined in discussion. The acceptable limits for calcium and magnesium for domestic use are 75 ppm and 30 ppm, respectively, in ground water. Whereas in case of non-availability of water sources, calcium up to 200 ppm could be accepted.
Conclusions
The results available for the Kaithal city indicate that groundwater problems are of diverse nature. The major problem is urban areas are related to increasing salinity, nitrate, fluoride and in some cases micro-pollutants.
Refernces
1. APHA, Standard methods for analysis of water and wastewater.18th Ed. American Public Health Association, Inc., Washington D C. (1992).
2. Meybeck M, Rev. Geol. Dyn. Geogr. Phys., (1997) 21: 215-246.
3. Patel, M. K., Mohanty, K., Tiwary, T.N. and Patel, T. K., Indian J. Env. Prot., (1994) 14(5): 373-379.
4. Mittal, S.K. and Verma, N, Indian J. Env. Prot., (1970) 17(6): 426-429.
5. Nawlakhe, W.G., Lutade, S.L., Patni, P.M. and Deshpande, L.S., Indian J. Env. Prot., (1995) 37(4): 278-284.
6. Shastri, S.C., Bakra, P. P. and Khan, J.I, Industry Environment and the Law, R 13SA Publishers, Jaipur (1996).






