Determination of nickel from water samples
K.N. Singh1 , S.N. Singh1 and G.S. Ojha1 *
1
Department of Chemistry,
S.G.R.(P.G.) College, Dobhi,
Jaunpur,
222149
India
2
Department of Chemistry,
R.S.K.D. (P.G.) College,
Jaunpur,
222 001
India
DOI: http://dx.doi.org/10.12944/CWE.3.1.28
The paper deals with the effect of nickel (A toxic heavy metal) concentration, and its determination from water and waste water by “Dimethyl Glyoxime Method” Nickel reacts with dimethyl glyoxime in the presence of an alkaline oxidizing agent to form a characteristic red colour complex which is measured visually and photometrically.
Copy the following to cite this article:
Singh K.N, Singh S.N, Ojha G.S. Determination of nickel from water samples. Curr World Environ 2008;3(1):181-184 DOI:http://dx.doi.org/10.12944/CWE.3.1.28
Copy the following to cite this URL:
Singh K.N, Singh S.N, Ojha G.S. Determination of nickel from water samples. Curr World Environ 2008;3(1):181-184. Available from: http://www.cwejournal.org/?p=801
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2008-02-08 |
|---|---|
| Accepted: | 2008-04-24 |
Introduction
"Pollution by metal is a great sin a man is doing against nature” (Kudesia 1985). Heavy metal in Ganga river have been reported Mathur etal (1987), Saikia etal.(1988), Prasad etal(1989), Chaudhary and Dutta (1980), Singh etal (1992). Out of 118 elements 83 are metals (Atomic number is above 23, mass density more than 5) in which 17 may be toxic at different concentration. Toxic metals change the biological structure and system in the body and they effect on enzyme system. Nickel a silvery metal, atomic weight 58.7, atomic no. 28, bp -275°C, m.p. 1450C, atomic radia 1.24Å ionic radia 0.72Å, it forms about 0.008% of the earth crust. Nickel ranks 24th among the element in the order of abundance on the earth. Its density is 8.92g/ c.c. Nickel ores are mainly of two types, Sulphide Pentlandie (FeNi) 9 S8, Chalcopyrite, Pyrrhotite and oxides (Laterites). It is used in electroplating and in various alloy preperation.e.g.Constantan (Cu), Nichrome (Cr. - Fe), German silver (Cu - Zn). It is also used in the production of stain less steel. Common brands of stainless steel contain 8% Ni and 18%Cr. It finds use as a catalyst and as a mordant in ceramic glasses. Emission of Nickel from fossil fuels is about 70,000-tonnes/ year. City air typically is contaminated with 0.03 - 0.12 g/m3 of Nickel and the daily intake via Lungs thus is about 0.3- 1.2 gm. Ni is an essential micronutrient for micro- organism and animals but not to plants. It is associated with the synthesis of vitamin B12. but is toxic at higher concentration. In animals, the toxic effects include dermatitis (‘Nickel - itch’) and respiratory disorders. Nickel inhibits various enzymes, including Maleic dehydrogenase, Cytochrome oxidase and Isocitrate dehydrogenase. "Nickel dusts are Carcinogenic". Nickel carbonyl, a volatile compound formed by the reaction of Nickel with Carbon monoxide is the most toxic of all nickel compounds, a carcinogenic agent. It is also found in many foodstuffs, but mainly in Tea, Cocoa, and Peanuts. Dietary intake of Nickel results from the use of cooking utensils made of stainless steel. An averages dietary intake of Nickel is 300 -600µg/day.
Experimental
Prepared stock Nickel solution by dissolving 447.9mgs.Nickel sulphate in one liter water. Prepared Nickel working solution of 100 ppm, from 1000ppm Nickel stock solution Sodium citrate solution; 25%an aqueous solution For preparing 0.05 N iodine solution firstly 20gs of KI is dissolved in 5ml water, then add 6.4 gs. of Iodine and diluted in to 1000ml water.
DMG Solution
1g DMG is mixed in 100ml. Liquor Ammonia and added 100-ml. distilled water.
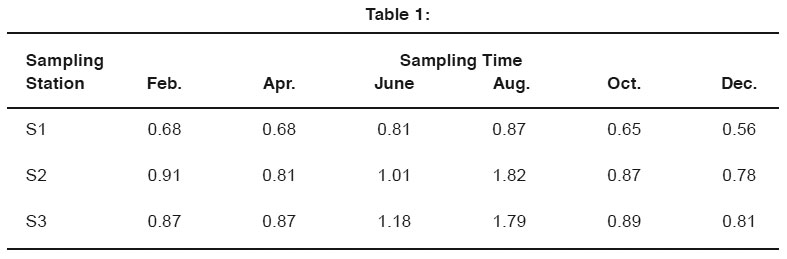 |
Table 1: Click here to view table |
Now took 100 ml. sample, blank and standard of Nickel, covering the range of 1, 0.5, 0.05 and 0.03 ppm respectively in different 50ml volumetric flasks. Added 20ml. 0.5 N HCl, 10 ml of aqueous sodium citrate solution and 2ml. of Iodine solution in each flask and shake well. After adding 4ml.of DMG solution in each flask made it up to the mark with distilled water and kept for 20 minutes. Compared the colour visually and photometrically using a spectrophotometer of 470nm and measured the mg Nickel equivalent of the observed optical density.
Results and Discussion
Toxicity of Nickel is believed to be very low. According to Indian Standard IS - 14543: 2004 maximum limit of Ni in drinking water is 0.02mg/l and the general standards for discharge of effluents contain maximum Ni, 3mgs/l. Ni is reported to be toxic to fish and other aquatic life. A bitter as tringent taste is produced by Ni on high concentration. Ni caused an appreciable fall in B.O.D. Thus 4mgs/l of Ni depresses BOD by about 17% and 42.5mgs/l caused a 50 % reduction.
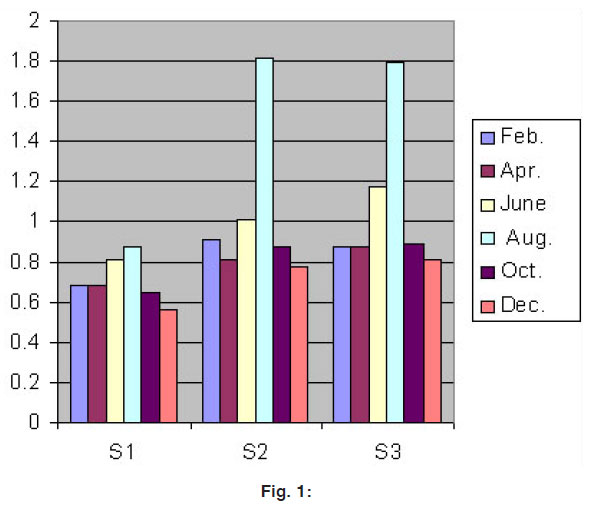 |
Figure 1: Click here to view figure |
In polluted water of Ganga river at Kanpur city maximum limit of Ni was found as 1.82 mg/l during rain season at (S2) Siddh Nath Ghat in Jajmau station. In Chandan Ghat its concentration was found 0.87mg/l and in Rani Ghat it was found 1.18 mg /l during analysis. From January 2006 - December 2006. At that time the minimum concentration was found 0.56mg/l at S1 sampling site in December 2006. Table1.0 shows the concentration of Ni in mg/l at three sampling station i.e. S1 (Chandan Ghat), S2 (Siddh Nath Ghat), and S3 (Rani Ghat) of river Ganga water at Kanpur. from January 2006 - December 2006.
It is clearly seen that there is some regular trend in the values of Ni concentration. These are highest during the rain season and lowest during the post monsoon. This can be explained on the basis of increasing in the volume of river in rain season as compared to the post monsoon when the dilution gives higher values of heavy metals in water.
During the period of January 2006 - December 2006 the Ni mg/l concentration is shown in figure 1.0 bimonthly variation at various studied sites.
Acknowledgements
The authors are thankful to the Principal, Dr. P.K. Singh S.G.R. (P.G.) College, Dobhi, Jaunpur for providing all necessary facilities for performing this type of work.
References
1. ABCM - SAC joint committee "Analyst" (1956) 81: 176
2. Feigi, F., "Spot test in Inorganic Analysis" Fifth edition, Elseveier Publishing Co.London (1958).
3. Sandel, E.B., "Calorimetric determination of traces of Metals”3rd edition interscience publishers, New York (1959)
4. Camp, T.R., "Water and its impurities" Reinhola publishing corporation, New York (1963)
5. Friberg, L.G.F Nordberg, "Hand Book of toxicology of Metals”. Elsevier. NY (1980)
6. Moore, J.W. and Ramamoorthy, S. "Heavy Metal in natural water" Applied monitoring and impact assessment, Springer Yerlag. NY (1984)
7. Krishnan Kannan "Fundamentals of Environmental Pollution." S. Chand and Company, New Delhi (1991)
8. IS 10500: Water Potability ’ “Drinking Water Standards” fourth addition, Buero of Indian Standard, Manak Bhawan, New Delhi (1999).
9. Kaushik, A.K. ET AL. Heavy Metals Pollution of River Yamuna in the industrially developing state of Haryana, Indian J.Environ. Hlth (2001) 43(4): 164-168.
10. Manivasakam, N., "Physico-Chemical Examination of Water, Sewage and Industrial effluents” 5th edition E (2005).
11.IS 13428, Annex L "Determination of Nickel in water and waste water" Buero of Indian Standard Manak Bhawan, New Delhi (2005).
12. DE, A.K. Environmental Chemistry, (2005).






