Assessment of heavy metals in ground water of Aurangabad Industrial areas
Sumantrao B. Bikkad1 and Sunil. R. Mirgane1 *
1
Department of chemistry,
J.E.S.College,
Jalna,
431 203
India
DOI: http://dx.doi.org/10.12944/CWE.3.1.19
Changes in the quality of ground water resources are related to presence and concentration of contaminants, especially heavy metals such as Arsenic, Cadmium, Chromium, Copper, Zinc Lead, Mercury and Nickel is known to have aneugenic effects. This study was carried out to assess the presence and concentration of heavy metal As, Cd, Cr, Cu, Zn, Pb, Hg, and Ni in ground water of Aurangabad industrial areas
Copy the following to cite this article:
Bikkad S.B, Mirgane S.R. Assessment of heavy metals in ground water of Aurangabad Industrial areas. Curr World Environ 2008;3(1):131-134 DOI:http://dx.doi.org/10.12944/CWE.3.1.19
Copy the following to cite this URL:
Bikkad S.B, Mirgane S.R. Assessment of heavy metals in ground water of Aurangabad Industrial areas. Curr World Environ 2008;3(1):131-134. Available from: http://www.cwejournal.org/?p=783
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2008-03-12 |
|---|---|
| Accepted: | 2008-05-17 |
Introduction
As in any country, the industrial growth in India has been currently boosted of to substantially enhance the economic growth. Without implementation of regulatory control measures for effluent quality. Industries in Chikalthana and waluj of Aurangabad industrial area gives off effluents rich in organic, inorganic1 contents as well as heavy metal have been found to result in toxic effect on near by environment .Heavy metal2 enter in human body by different path way and cause harmful effect.3
Present study was under taken keeping in view significance of ground water metal characterization and source identification constitutes important aspect of study dealing with environmental degradation.
Material and Methods
Ground water samples were collected from different bore wells present in industrial areas of Aurangabad.Water of which is being used by public for drinking and domestic purposes. In all eight sample were collected in one liter polythene screw bottle from each place in July 2007 and December 2007 .Bottle were thoroughly cleaned with 50 % Nitric acid followed by thrice washing with double distilled water. The samples were filter immediately through 45µm.milli pore membrane filture and were acidified with 5 ml HNO per liter.
Sampling Sites
S-1. Powerloom area - Chikalthana MIDC.
S-2. Pandharpur Village Walunj MIDC.
S-3. Ghanegaon near Good Year Tyre Walunj MIDC.
S-4. Jogeshwari Village Walunj MIDC.
S-5. Ranjangaon Hanuman Nagar Walunj MIDC.
S-6. Z.P. School Ranjangaon Walunj MIDC.
S-7. Pandharpur Grampanchayat Walunj MIDC.
S-8. Near Deogiri Chemicals Chikalthana MIDC.
Prior to analysis all sample were subjected to controlled Nitric acid digestion4 on hot plate until clear light colored solution is obtained. Filtrate was cooled, diluted and mixed thoroughly. All the reagents used were of A.R grade.
Heavy metal analysis was done with Atomic Absorption Spectrophotometer, GBC AVANTA PM -10 Australia by direct aspiration method.
Results and Discussion
Result for analysis of heavy metal content of ground water sample collected from different place are summarized as in Table1.
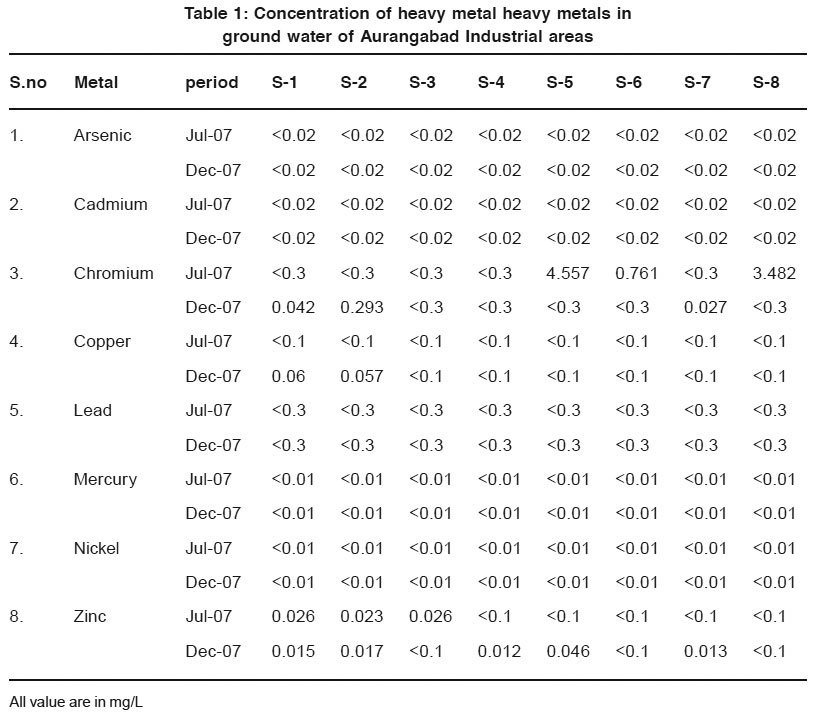 |
Table 1: Concentration of heavy metal heavy metals in ground water of Aurangabad Industrial areas Click here to view table |
Arsenic
Arsenic in drinking water is highly undesirable because of its toxicity. In present work Arsenic is found to be below 0.02 mg/L which is below permissible limit of WHO5 and BIS6 for drinking water.
Cadmium
Cadmium is conceder to be one among the environmentally hazardous metal because it has higher toxicity greater capability of accumulation and retention in the body of organism including human. In present study Cadmium concentration found to be below 0.02 mg/L the value is higher than WHO recommended for drinking water. This may be due to west of electroplating industries percolated into ground water.
Chromium
Chromium is naturally occurring element and essential for good health i.e. synthesis of fat from glucose and also for the oxidation of fat to Carbondioxide7, 8 Chromium hexavalant is more toxic than chromium trivalent. Higher value of Chromium at S-5 is 4.557 mg/L ,at S-8 is 3.482 mg/L and S-6 is 0.761 mg/L .May be due to discharges of west from paint ,dyes and Ceramic industries. Near sampling station. At remaining sites concentration of Cr is within desirable limit of WHO.
Mercury
Mercury and Mercuric compound are considered to be highly toxic to human being. Prolonged Mercury ingestion can cause kidney and brain damage. Hg concentration of all site found to higher than drinking water limit of WHO and BIS.
Copper
Copper is essential metal for all living organisms because of it’s key role in many enzymatic reaction. However at high concentration it become essentially pollutant,9 in present work concentration of copper is below 0.1 mg/L at all sites which is above permissible limit of BIS. This high dose of copper through water in human being may cause epigastria burning, vomiting and diarrhea.
Zinc
Zinc is an essential beneficial metal for human being it enters in water supply and water body from deterioration of galvanized iron and dezincification of brass .the maximum value of Zinc ion ground water is 0.1 mg/L. and minimum of 0.021 mg/L. which are within limit of BIS.
Lead
Lead is highly toxic, high level explore of Lead content in potable water may cause cancer and blood pressure. Increase in lead content is indicative of an input from rain water runoff, effluents and house hold sewage sanitation in study area .In the sample concentration of Lead found to be below 0.3 mg/L which is above permissible limit of BIS and WHO.
Nickel
Number of studies have pointed out that Nickel has nutrient importance, where it is engaged in the composition of nucleic acid .how ever nickel related health effect such as cardiovascular has been reported. In present investigation concentration of Nickel below permissible limit of WHO i.e. 0.02 mg/L.
Conclusion
Most of heavy metal contaminants are abundantly present in water sample .concentration of these metal are abnormally high and restrict the use of water for many domestic purpose. Site factor are also important in deciding the probable in metal content in bore well water it was found that amount of Cr and Pb is alarmingly high there fore it is suggested that water from these bore well should be totally abandoned and alternative arrangement for water supply be made available to the local people.
Acknowledgements
The author would like to acknowledge the help rendered by Mumbai Waste Management Limeted, Taloja. For providing necessary fascilities to under take this investigation.
References
1. M.B.Ubale,Farooqui Mazher,Arif Pathan, Md. Zaheer Ahamed ,Dhule D.G. Oriental journal of chemistry. (2001) 17(2): 347-348.
2. S.Mansoor, Munir.H.Shah. N.Shaheen, A.Khalique,M.Jaffar.Journal of Hazardous Materials. (2006) A137: 31-37.
3. S.Krishnamurthy. J. Chem. Edu. (1992)69: 347.
4. APHA. Standard Method for the Examination of Waste and Waste Water .16th Edn.American Public Health Association, Washington D.C (1985).
5. World Health Organisation ,Guidlines for Drinking Water –Quality ,Geneva,3 rd Edn. 1 (2004).
6. BIS:10500: Drinking Water-Spesification, First Revision ,Bureau of Indian Standards, New Delhi (1991).
7. G. Phillips and R. Russo,U.S.EPA. (1978) 60013-78.
8. B.Milane, in :CA.Burtis and E.R. Ashwood [Eds], Text Book of Clinical Chemistry,W.B. Sounders Company, Tokyo-London, (1999) 30: 1029.
9. Chi-ManLeung,Jiu Jimmy Jiao,Water Reserch. (2004) 40: 753-767.
10. Moore.James,N.and Rammoorthy, S., Heavy Metal in Natural Water;Applied Monitoring and Impact Assessment,Springer- Verlag,New York (1984) 28-246.
11. E.F.Pane,J.G.Richards and C.M. Wood. Aquat.Toxical. (2003) 63:65.






