Analytical studies of some ground water in Sailu tehsil of Parbhani district
D.U. Thombal1 , R.U. Ambhure1 and S.R. Mirgane1 *
1
Department of Chemistry,
Jalna Education Societys, R.G. Bagdia Arts,
S.B.Lakhotia Commerce and R.Bezonji Science, College,
Jalna,
431 203
India
DOI: http://dx.doi.org/10.12944/CWE.3.1.25
Analytical studies of thirty groundwater samples from diffrent sites in sailu tehsil was carried out during the month of April-2007. The water quality parameter like temperature, pH,electrical conductivity, total dissolved solids (TDS), total alkalinity (TA), total hardness (TH), chlorides (Cl-),Sulphates (SO42-), Calcium (Ca2+), Magnesim (Mg2+), Sodium (Na+), Potassium (K+),dissolved oxygen (DO) and turbidity (TUB) were studuied and out come of the results were disscoused.
Copy the following to cite this article:
Thombal D.U, Ambhure R.U, Mirgane S.R. Analytical studies of some ground water in Sailu tehsil of Parbhani district. Curr World Environ 2008;3(1):161-165 DOI:http://dx.doi.org/10.12944/CWE.3.1.25
Copy the following to cite this URL:
Thombal D.U, Ambhure R.U, Mirgane S.R. Analytical studies of some ground water in Sailu tehsil of Parbhani district. Curr World Environ 2008;3(1):161-165. Available from: http://www.cwejournal.org/?p=795
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2008-03-12 |
|---|---|
| Accepted: | 2008-05-17 |
Introduction
Sailu is considered to be the oldest and religious city in parbhani district of Marathwada region in Maharashtra, Sailu city is situated near Dudhana river. A Femous Temple of "Keshavraj Babasaheb Maharaj" is situated in middle of sailu city. Who was Guru of Shirdis Sai baba.
The residents of Sailu tehsil usually use water form bore-well for drinking and domestic purposes.There is a huge variation in the concentration of dirrrent species due to factors like depth,diffrent land ,under groundwater conditions, rain condition s etc. The present work attempts to evaluate the quality of groundwater in sailu Tehsil of Parbhani district for potability.
Material and Methods
In the Present study thirty groundwater (borewell. samples were collected from diffrent sites of Sailu tehsil in brown glass bottles with necessary precautions and preserved as per the recommended procedures.¹
All the chemicals used were of AR grade, glass ware used were of ' A' grade.Double distilled water was used through out the work to prepare standard solution²
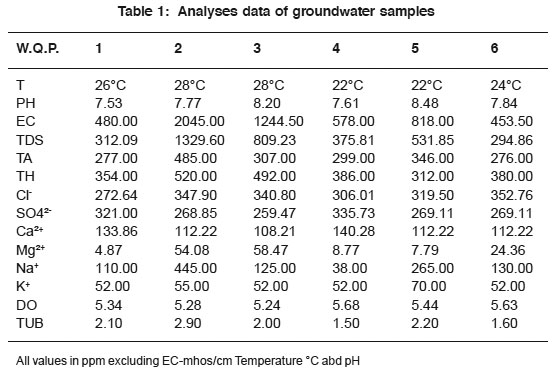 |
Table 1: Analyses data of groundwater samples Click here to view table |
The water quality parimeters considered for the examination in this study are Temperature by precision thermameter (110°c., pH3 by digital PH meter (Model No.LI 613 Elico digital PH meter.,electrical conductivity by using Elico digital conductivity meter (Model No.LICM180.,4 total dissolved solids by evaporation method at 105 -110° c5-6 total alkalinity by standard procedure,7 total hardness by comlexometric titration method,8 Chloride by argentometry,9 Sulphate by nephelometry, Calcium and Magnesium by complexometry method, sodium and potassium by flamephotometer (systronics, mediflame model No. 127, India, Dissolved oxygen by winkler's Iodometric method10 and turbidity by turbidimeter.
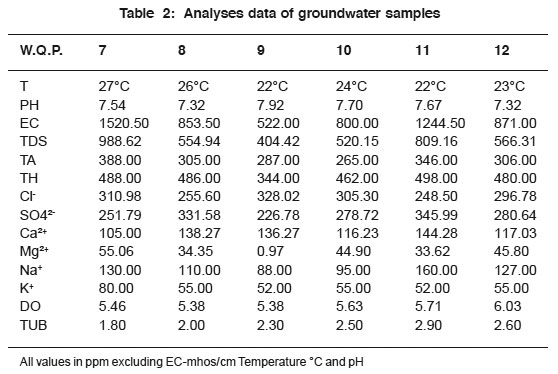 |
Table 2: Analyses data of groundwater samples Click here to view table |
Results and Discussion
Thirty ground samples were collected from diffrent sites of sailu tehsil. The results indicates that the quality of ground water has wide variation which is reflucted by the values of electrical conductivity, Chloride, Sulphate, Calcium and Magnesium etc.
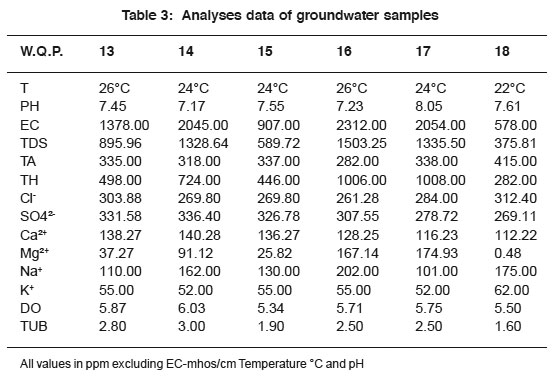 |
Table 3: Analyses data of groundwater samples Click here to view table |
pH acts as index to determine the extent of pollution, chemical and biological reactions are direatly dependent upon the pH of water system. In the present study pH ranged from 7.17 to 8.48 which lies in the range preseribed by WHO11 Electrical conductivity Value, in present study renged from 435.50 to 3112 all were found to be well above the permissible limit and are quite unfit for drinking.
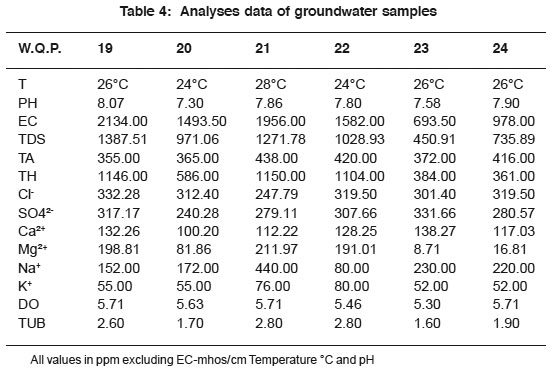 |
Table 4: Analyses data of groundwater samples Click here to view table |
Drinking water quality is affected by the presence of soluble salts.Total dissolved solid (TDS. is an important parameter in drinking water quality standard. It developes a perticular test to the water and at higher concertration redudces its potability, plants are also severely affected by higher values of TDS in irrigation water. TDS value of study area ranges from 294.86 to 2023.40 ppm. The high TDS level (7500. will result in the excessive scaling in water distrubution system12.Total alkalinity (T.A.. were found to be in the ranges 265 to 485 ppm. All samples are above the permissible limit precribed by ICMR.13 The higher alkalinity of groundwater owing to the presence of bicarbonates and trace amount of carbonate14 and hydroxide salts.15 Water hardness is traditional measure of the capacity of water to reacts with soap Hardwater couse horrific effects in digestive system moreover, the possibility of forming calcium oxalate crystals in uninary track has been ascertained.The hardness value of groundwater in the present study area ranges from 282 to 1150 ppm.
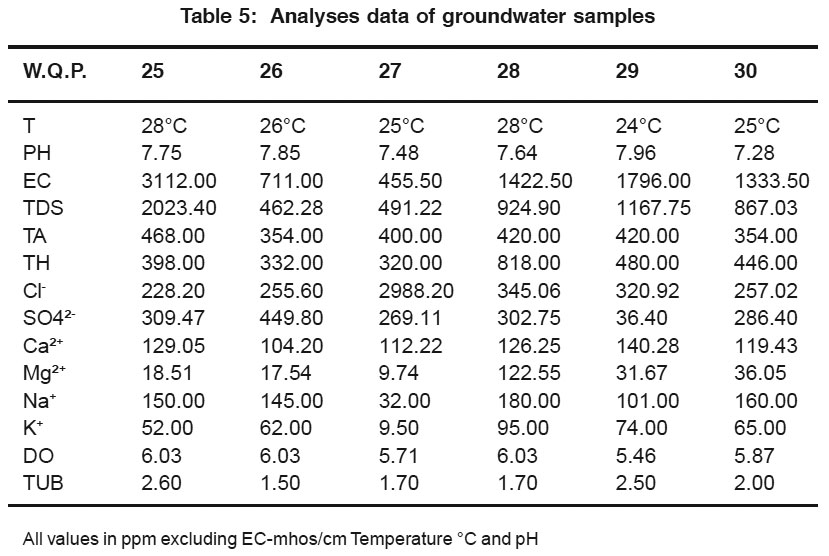 |
Table 5: Analyses data of groundwater samples Click here to view table |
Chloride content were found to be ranging from 247.79 to 347.90 Chloride in the maximum sites was found to be well above permissible limit which may be due to the absence of proper drainage system in the study area. According to ISI permissible limit of sulphate concentration is 150 ppm, Beyond this limit sulphate causes gastro intestinal iritation and can have laxative effect in presence of Mg and Na.sulphate renges from 240.28 to 449.90 ppm. in the present work Calcium in present study varies from 100.20 to 144.28 ,High concentration of calcium may be due to leaching of soil deposit of limestone, dolmite, gypsum, pypsiferous materials, silicious sand into ground water. Magnesiun is an essential mineral for the living body, High concentration of mg couses nausea, musular weakness and paralysis in human body when it reaches up to the level of about 400 mg/L. In this area, magnesium concentration ranged form 0.48 to 211.97 ppm. Sodium and potassium enters in drinking water from natural geological sources, detergents, domestic industrial dischages and minig wastes. In the present work. sodium concentration varies from 32 to 445 and potassium varies from 9.5 to 95 ppm. Oxygen is disolved in most water in varying concentrations. Solubility of oxygen depends on temperature, pressure and salinity of water. It is essential to the life of fish and other aquatic organisms. In the present study Dissolved Oxygen. ranges from 5.24 to 6.03 ppm.Turbidity is an important parameter for characterising water quality.In the present study turbidity varies from 1.5 to 3.00 NTU. These values are well below the permissible limit as per WHO (5 NTU.11
Acknowledgements
We are thankful to principal Dr.R.S.Agrawal, Dr.S.M.Deshpande Head of department, Jalna Education Socitys, R.G.Bagedia Arts, S.B. Lakhotia Commerce and R.Bezonji Science, College , Jalna for providing necessary facilities and help for the present work.
References
- American Society for Testing materials, Annual Book of ASTM Standard,Part-23, ASTM - Phifadelphia (1972).
- Text Book of Quantitative Inorganic Analysis, 2nd edn , A.I.Vogel, 191, Longman and Green Co, London (1985).
- R.G.Bates,Determation theroy of pH and practice, 2nd, Edn , wiley, New York,(1973).
- R.A. Robinson and R.H.Stokes, Electrolytic solution, 2nd, Edn, Academic press, New York, (1959).
- Physico-chemical examination of water and waste water and Industrial Effluents, N. Manivaskam, pragati prakashan, Meerut India.
- C.S. Howard, Determination of total dissolved solid in water Analysis, Introduction to engineering chemistry, anal Edn, (1933) 5: 4.
- Shell-Eitra, Encyclopedia of industrial Ami Chemical analysis, (2000) 19: 1123.
- APHA, Standard method's for the examination of water and waste water 16th edn, Washingaton D.C. (1975).
- Volumetric Analysis, 2 nd Edn vol -2 I.M.kolthoff and V.A.stenger, Interscience Publishers, New York.
- S. Hooda and S. Kaur, Laboratory manual for Environment Chemistry, S. Chand and company limited (1999).
- World Health organization, Guidlines for Drinking water Quality, Recomendation of WHO, Geneva (1984 and 1996) 1: 1-130.
- D.P. Tihansky, Water Resource, Res, (1974) 10(2): 145-149.
- ICMR Manual of standards of Quality for Drinking water supplies, spl . Rep. S.No. 44, ICMR, New Delhi India (1975).
- P Zuddas and F. Podda, App. Geochem, (2005)​​​​​​​ 20: 507-517.
- Physico-chemical Examination of water and waste water and Industrial Effluents, N. Manivaskam, Pragati Prakashan, Meerut India.






