Bacteria, biochemical changes and sensory characterization of sorghum lager beer production
B. Boboye1 *
1
Department of Microbiology,
Federal University of Technology,
Akure,
P. M. B. 704
Ondo State
Nigeria
DOI: http://dx.doi.org/10.12944/CWE.2.2.02
This study was conducted to identify bacteria encountered and some biochemical changes which occurred during the production of sorghum lager beer. Sensory evaluation was also carried out to compare physical properties of the beer with industrially manufactured lager beer. Erwinia, Enterobacter, Bacillus, Flavobacterium, Streptococcus, Acetobacter Lactobacillus, Acinetobacter and Aerococcus species were isolated during the production. Pasteurised beer did not contain any microbe. Ethanol content and total acidity increased while pH, specific gravity, apparent extract and total carbohydrate contents decreased during the fermentation of the sorghum wort with final values of 3.62%, 0.16%, 4.2, 1.01600, 2.70 and 38.0 mg/l respectively. At 5% level of significance, the finished lager beer was generally acceptable to the panellists. It scored unsatisfactory in colour and consistency but similar in taste and odour to the industrially manufactured lager beers. Thus, the use of sorghum for the production of lager beer without adjunct could be practised.
Copy the following to cite this article:
Boboye B. Bacteria, biochemical changes and sensory characterization of sorghum lager beer production. Curr World Environ 2007;2(2):107-114 DOI:http://dx.doi.org/10.12944/CWE.2.2.02
Copy the following to cite this URL:
Boboye B. Bacteria, biochemical changes and sensory characterization of sorghum lager beer production. Curr World Environ 2007;2(2):107-114. Available from: http://www.cwejournal.org/?p=655
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2007-07-12 |
|---|---|
| Accepted: | 2007-09-02 |
Introduction
Brewing, the preparation of beer from carbohydrate material by means of the action of yeasts is a few decades old in Nigeria. It started in 1949 with the establishment of Nigerian breweries limited in Ibadan and since then malted barley has been the major source of carbohydrate used. Corn, rice, cassava, millet and sorghum are other sources of carbohydrate used. They are commonly used as adjunct and where utilized wholly they were used with less success relative to malted barley. Merit lager beer was made from 100% corn by Guiness brewery and Rex lager beer from 40% sorghum and 60% malted barley by Nigerian breweries limited, Lagos, Nigeria. Rajagopal1 reported that lager beer brewed from cassava was not acceptable to many beer consumers. Virtually all these beers are not in the market today. This is mainly because quality of the final product of beer brewing depends solely on the initial composition of the raw materials.
The grains should possess a high diastatic power, low fat content, fairly low protein content, high starch and vitamin content.2 Barley is a cereal which satisfies this condition. The problem associated with the production of Merit beer is that corn has a low diastatic power and thus requires external enzymes to effect good saccharification.3 This factor invariably led to higher sale price of the Merit than Harp lager beer made from barley. Cassava contains hydrocyanic acid, the content of which can be reduced during processing. This however will increase cost of production. Also, low percentage of cassava is available for brewing beer because it is consumed in large quantity in Nigeria.
Millet is similar to sorghum but smaller in size. It is commonly taken as food in Nigeria. Sorghum compares well in chemical composition to barley. It contains 8-15% protein, 2-5% fat, 68-74% carbohydrate, 1-3% fibre and 1.5-2.0% ash.4 Malted sorghum possesses inherent amylase necessary to bring about liquefaction of mash and 20-40% saccharification.5 This property coupled with the use of improved method of malting can lead to achievement of better saccharification.2
Sorghum (Sorghum bicolor (L) Moench) which belongs to the family Graminae is the most widely grown Nigerian cereal especially in the northern part of the country with an average annual production of five million tonnes.6 Sorghum is used for the traditional production of local alcoholic drinks such as pito, pap and burukutu. It is equally used as an adjunct with other cereals for the manufacture of sparkling and clear lager beer.7,8 The cereal is acceptable for the production of African beer.9
The expanding brewing industries in Nigeria needs a good cheaper source of carbohydrate than malted barley particularly when the increasing population, slow economic growth and development of the country are considered. Sorghum may be a good alternative. In order to make this a success it is important to compliment production of the lager beer with quality tests which will lead to understand the source of microbial contaminants and measures required to control them. Therefore this research was aimed to identify bacteria and some biochemical changes associated with laboratory manufacture of lager beer from sorghum. Comparative analyses were conducted to determine possible consumers preference.
Material and Methods
Brewing Ingredients
Sorghum grains (Sorghum bicolor (L) Moench) was obtained from “Oba” market, Akure, Ondo State, Nigeria. Hop pellets and yeast (Saccharomyces carlbergensis) were kindly supplied by Bendel and Standard Breweries in Benin and Ibadan towns of Nigeria respectively.
Brewing
The method of Skinner10 was used for malting. Sorghum grains (400 g) were steeped in tap water for 16 h at 28°C and spread on a washed concrete floor. The grains were turned and sprinkled with water three times daily at 8 h interval for 5 days at a temperature range between 25-35°C. Malting was checked by chewing few grains on each successive day until a sweet taste developed (usually the fifth day) when the plumules measured 3.8 cm. Malted grains were dried.
Grist was made from the malted sorghum grains by grinding them in a blender mill to a fairly coarse texture. The grist (240 g) was sieved and stirred with warm water (860 ml) at 35°C, CaCl2 (0.28 g) and formalin (0.1 ml). It was mashed in an intensive mashing process with three-stage decoction system. Saccharification test was carried out by the addition of few drops of iodine solution (0.2N) to the mash sample. This was to check conversion of starch to sugar. Change in colour to light yellow indicated a good saccharification. The mash was boiled to inactivate the enzyme and filtered through Whatman filter paper No.1 using a filter pump. Sweet wort was obtained and boiled for 1.5 h during which hop pellets (1.5g) and HCl (0.2 ml) were added. This constituted the bitter wort which was cooled to 5oC and pitched.
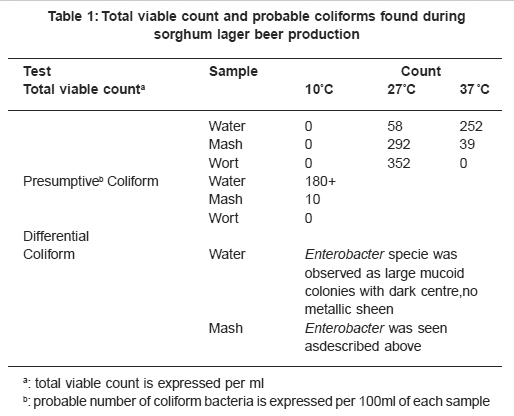 |
Table 1: Total viable count and probable coliforms found during sorghum lager beer production Click here to view table |
Pitching was carried out by adding to the bitter wort, Saccharomyces carlbergensis (75g) previously suspended in 50 ml sterile distilled water. Primary fermentation was then done at 10°C for a week to obtain ‘young’ beer. It was decanted from the bottom sedimented yeast and secondary fermentation was allowed to set in during which the remain suspended yeast continued to produce alcohol from sugar. This ageing period took two weeks. The matured lager beer was then filtered into sterile bottles through hyflo kieselguhr and Whatman filter paper No.1 with a filter pump in a disinfected enclosed chamber. Sugar (1g) and dried yeast (0.5g) were added to the filtered beer for carbonation. It was corked, agitated, left for one hour and pasteurized at 68oC for 30 minutes.
Bacterial and Biochemical Analyses
Samples were collected at various stages of the brewing for bacterial and biochemical analyses. Water, mash and wort were analysed for bacterial total viable count using pour plate method with nutrient agar (NA), Czapek dox agar (CDA) and wort agar (WA). They were incubated at 10, 27 and 37°C for 48 h. Presumptive and differential coliform tests were carried out on the samplesby using MacConkey broth and Eosin methylene blue agar according to standard procedure.12 Inoculated media were incubated at 37°C for 48 h. Malted sorghum, hops, cultured yeast, unpasteurized and pasteurised beer were also inoculated onto NA, CDA and WA and incubated at 37°C for 24 h. Bacteria were subcultured to obtain pure isolates.
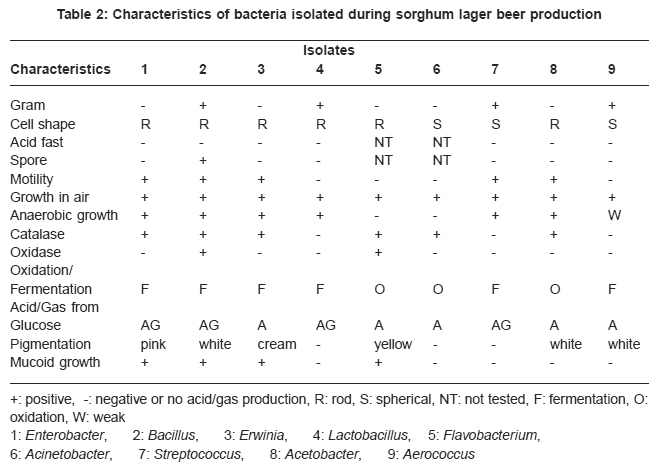 |
Table 2: Characteristics of bacteria isolated during sorghum lager beer production Click here to view table |
Identification tests were done on the bacteria isolated from various samples using the methods of Cowan and Steel,11 Cruicshank et al.12 and Delaat.13 The tests carried out were gram stain, shape, acid-fast stain (Ziehl-Neelsen’s method), spore stain, motility, aerobic and anaerobic growth, catalase, oxidase, oxidation-fermentation (OF) of glucose, production of acid and gas from carbohydrate (glucose), pigmentation and mucoid growth.
Chemical analyses were carried out according to the methods of Egan et al.,14 and Friburg.15 Fermenting wort samples were collected for analyses on 0 to 7 and 21 days of fermentation. Hydrogen ion concentration of the sample was measured with a pH meter (PW 9818 Philips model) after standardization. A colour comparator (Helige model with European brewing convention scale cover) was used to measure colour value of the beer. The beer sample was poured into a 26 ml cuvette, placed in the comparator and matched with the colour disc until an appropriate colour was obtained. Specific gravity bottle was filled with the sample, incubated for 30 min at 20°C and weighed. This procedure was repeated with distilled water. The specific gravity was calculated as the weight of the sample multiplied by the reciprocal of the weight of water. Sample (5 ml) was diluted in boiled distilled water (200 ml) and titrated against standardized NaOH (0.1N) with 7 drops of phenolphthalein as indicator. Acidity was calculated as dilution factor multiplied by equivalent of acid, normality and titre value of NaOH divided by volume of the sample. It was expressed in percentage. Hundred millilitres of the wort sample with 40 ml distilled water was distilled in a volumetric flask until 95 ml distillate was collected. This was made up to 100ml with distilled water. Specific gravity was determined as stated above. A standard alcohol content table was referred to in order to estimate the alcohol content in percentage.
Spectrophotometric method was employed to measure the total carbohydrate content of the wort. The sample was diluted 1000 times, mixed with 5% phenol solution (1 ml). Concentrated sulphuric acid (specific gravity of 1.84) (5 ml) was added and left for 10 min at room temperature. It was shaken thoroughly and put in a water bath maintained at 28°C for 15 min. Absorbance of developed yellow colour was measured at 490 nm in a spectrophotometer (LKB Biochrom, Lutrospec II). A standard curve was previously constructed with a series of glucose concentrations ranging from 0 to 100 mg/litre. Total carbohydrate value was read off from the standard curve. Apparent extract content was measured by using brix hydrometer and expressed as °plato. Standard Micro-kjedahl method was used with 1.5 g dried beer sample. Nitrogen content was multiplied with 6.25 to obtain protein content.
Nine randomly selected judges compared the test samples with commercial beers in colour, consistency (body), taste, odour and overall acceptability. The total scores were compared at 5% level of significance.
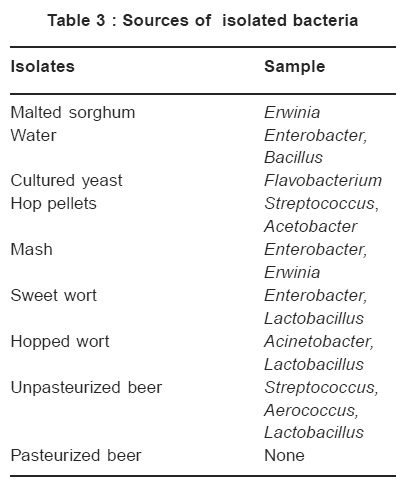 |
Table 3 : Sources of isolated bacteria Click here to view table |
Results and Discussion
Characteristics of Bacterial Isolates
The total viable count of bacteria in water was higher on plates incubated at 37°C and 27°C than at 10°C. It was higher at 27°C than 37°C for mash and wort samples Table 1. This shows that the samples were contaminated with mesohpilic bacteria. Presumptive and differential tests showed that the gram positive and negative aerobic microbes while some grew in the absence of oxygen Table 2. All but Bacillus were non-spore formers. None of them was acid fast. They showed various reactions to catalase and oxidase tests. All the bacteria produced acid from glucose. The Acetobacter however oxidized glucose but did not ferment it.
Coliforms, Bacillus and Erwinia were found in water and malted sorghum Table 3. Hop pellets, sweet and hopped worts contained Streptococcus, Acetobacter, Enterobacter, Lactobacillus and Acinetobacter. Enterobacter was transferred to mash and sweet wort from preceeding water and mash respectively. Presence of coliforms in the water sample may be because the water was inadequately treated or was contaminated. Coliform bacteria are hop tolerant and can be dangerous when wort has to be stored unpitched for sometime; they develop quite rapidly with the production of celery-like or phenolic odours and cause unpleasant flavour in weak beer.16 The Enterobacter can be killed by boiling of the wort. Bacillus is an endospore producer. This microbe was not encountered in intermediate and final products because of high water contents of the products coupled with heat treatment. Spores of the bacteria showed low resistance to heat in the presence of high water content.17
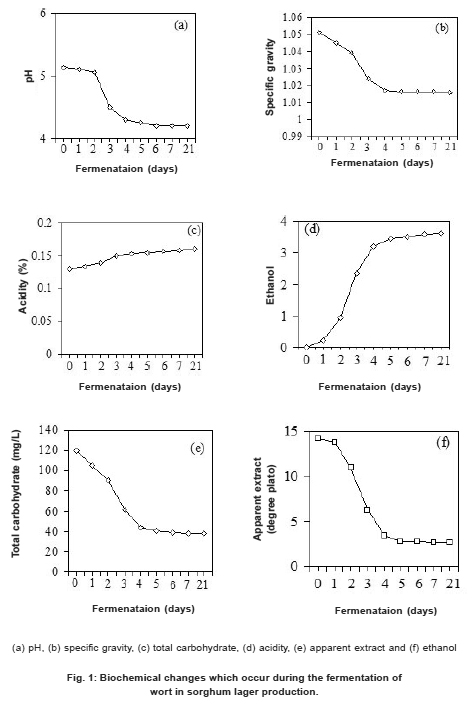 |
Figure 1: Biochemical changes which occur during the fermentation of wort in sorghum lager production. Click here to view figure |
Erwinia is a plant pathogen. Its association with mash must have resulted from sorghum grains contaminated from the field. Flavobacterium was found on cultured yeast. The organism is common with pitching yeast.18 This is because it is hop insensitive and it grows best at pH 5.0 and about 32oC producing parsnip smell.16 It contaminates hopped wort easily.
Unpasteurized beer contained Aerococcus, Streptococcus and Lactobacillus. Streptococcus was transferred from hop pellets. Streptococcus, coliforms, Acetobacter and Lactobacillus are common with the production of malted barley beer16, 18 and burukutu, pito and palmwine.19 Streptococcus and Lactobacillus are fermentative and could impact sharp taste in beer left unpasteurized for a length of time due to acid production.18,20 Streptococcus (Beer sarcina) causes “sarcina” sickness due to production of diacetyl honey-like odour. Lactobacillus is a non-sporulating rod which also lowers pH and could produce ropiness, silky turbidity and unpleasant flavour in Beers.16,18 Acetobacter in finished beer converts ethanol to acetic acid thus lowers pH of the beer and can cause pellicle formation. Acinetobacter and Aerococcus are not common with beer production. Their presence in hopped wort and unpasteurized beer must be due to surrounding air contamination.
Pasteurized beer did not contain any microbe. The microorganisms encountered in the earlier stages have been destroyed by the heat of pasteurization. Thus, the finished product is safe for human consumption. Some of the bacteria isolates encountered in this work are not common with breweries. The commonly associated bacteria with beer producing factories are Lactobacillus, Streptococcus, Acetobacter, Flavobacterium and Enterobacter; all other isolated bacteria are contaminants particularly catalase positive cocci. These bacteria are non-spore formers with the exception of Bacillus. Vegetative forms of bacteria are heat labile than the spore forming ones.
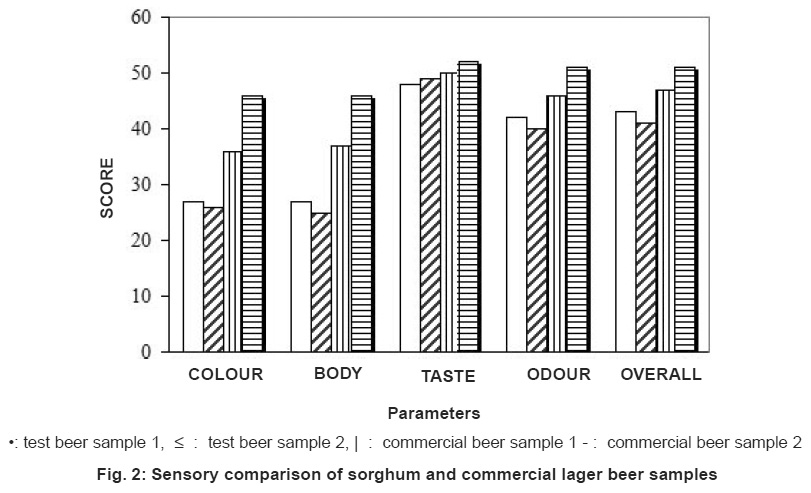 |
Figure 2: Sensory comparison of sorghum and commercial lager beer samples Click here to view figure |
Biochemical and Sensory Properties
Hydrogen ion concentration, specific gravity, apparent extract and total carbohydrate contents decreased while amount of ethanol and total acidity increased with increased length of fermentation of the bitter wort Fig. 1. The finished lager beer had a pH of 4.2, specific gravity of 1.01600, 2.70 oplato with 38 mg/l of total carbohydrate. The ethanol content changed from 0 to 3.62% and the total titratable acidity increased from 0.13 to 0.16%. The final protein content of the finished test beer was 0.42% which is higher than that of beer made from malted barley mixed with sorghum (0.34%). Colour value of the sorghum lager beer (3.9) agrees with that of American Society of Brewery Chemists which is 3.9.21
The result of the multiple comparison test showed that there are differences and similarities between the brewed sorghum beer and the commercial ones. The panel judges noted an appreciable difference in colour and body (consistency) between the beers. Commercial lager beers rated better than the test sample. This may be due to the natural colour and other nutrient components of the sorghum, extra yeast added for carbonation and filtration method used. However, there was no difference in taste and odour of the beers. In general the tested lager beer was acceptable.
Conclusion
Some of the bacteria associated with this beer production have been found in the manufacture of barley beer. Thus the microbes reported in this study will not pose serious problem if routine cleaning and disinfection of all processing equipments, enlightenment of workers on personal hygiene and practice of asepsis during inoculation are ensured. The relative difference noted in the samples could be reduced appreciably or prevented by the use of sophisticated equipment and direct carbonation with carbon dioxide as used in breweries and sorghum with low protein content or a mixture of high and low protein sorghum grains. This will reduce haziness and increase shelf life of the beer. Therefore the use of 100% sorghum in brewing industries seems encouraging particularly when the same procedure used in the making of barley beer can be employed.
Acknowledgements
The author is grateful to Federal University of Technology, Akure, Bendel and Standard Breweries in Nigeria for the provision of materials and equipments used for this research study.
References
-
Rajagopal, M. V., Production of beer from Cassava. J. Food Sci. (1977) 42: 532.
-
Chiba, H.; Fujimaki, M.; Iwai, K.; Mitsuda, A. and Morita, Y., Recent progress in beer production. Proceedings of the fifth International Congress of Food Science and Technology, (1979) 1: 290-295.
-
Omonike, A. O., The utilization of sorghum as a brewing adjunct in Nigeria. A Seminar Paper presented to Brewers’s Association of Nigeria in (1978) 1978.
-
Purseglove, J. W., Chemical composition of sorghum grains. In: Tropical Crops Monocotyledons 1. Longman group Limited publisher, London. (1972) 263-275.
-
Novellie, L., Kaffircorn malting and brewing. Studies IV: Occurrence of b-amylase in Kaffir malts. J. Sci. Food Agric., (1960) 11: 457.
-
Allordiah, E. C., Exploiting cereal based raw materials for industrial development. A paper presented at the symposium of the Nigerian Institute of Food Science and Technology, Western Chapter, held at the Federal University of Technology, Akure, Nigeria. (1986).
-
Dhamija, S. S. and Singh, D. P., Adjuncts in brewing 1, Bajra and sorghum. J. Food Sc. (1978) 15: 197-200.
-
Anichie, G. N., Studies on the brewing of lager beer from sorghum and other local Nigerian raw materials. Ph. D. Thesis, University of Nsukka, Nigeria. (1982).
-
Okafor, N. and Anichie, G. N., Studies on the brewing of lager beer from Nigerian sorghum. J. Food Sci. Tech., (1987) 24: 131-134.
-
Skinner, R. Tropical lager brewing with sorghum malt. Brew Dist. Int. (1976) 6(8): 26-27.
-
Cowan, S.T. and Steel, K.J. Manual for the Identification of Medical Bacteria. Revised 2nd Ed. Cambridge University Press, London, (1974) 45-180.
-
Cruickshank, R., Duguid, J.P. and Swain, R. H. A. Bacteriology of water. In: Medical Microbiology, 11th Ed., Churchill Livingstone Publisher, Edinburgh (Teviot place), (1968).
-
Delaat, A. N. C. Staining and related methods: Spore staining. In: Microbiology for Allied Professions. Kimpton Publishers, London, 135, 1975.
-
Egan, H., Kirk, R. S. and Sawyer, R. Fermented products. Pearson’s Chemical Analysis of Foods. 8th Edition. Churchchill Livingstone Lublisher, Leith Walk, Edinburgh, (1981) 345-383.
-
Friburg, I. B., Instructions for Analysis. Fritz Hellige Company, GmBH D-7800, Germany. (1977).
-
Ault, R. G. and Newton, R. Spoilage microorganisms in brewing. In: Modern Brewing Technology. Edited by W. P. K. Findlay. Cleveland: CRC Press, Chapter 9. (1971).
-
Brock, T. D., Smith, D. W. and Madigan, M. T. Biology of Microorganisms. Prentice-Hall International, New Jersey, (1984) 24.
-
Harrison, J., Webb, T. J. and Martin, P. A. The rapid detection of brewery spoilage microorganisms. J. Inst. Brew., (1974) 80: 390-398.
-
Ogbonna, C.I.C., Onwuliri, C.O.E., Akueshi, C. O. and Yilzung, F. D. The microorganisms associated with locally prepared Burukutu, Pito and Palmwine. Nigerian J. Biotech., (1983) 1: 109-120.
-
Pelczar, M.J., Reid, R.D. and Chan, E.C.S. Environmental and Industrial Microbiology. In: Microbiology, 4th ed., Tata McGraw-Hill publisher Company Limited, New York. (1977).
- Tehinse, J.F. and Ogundiwin, J.O. A study of some quality characteristics of OTIKA, a traditional alcohol beverage from guinea corn. Proceedings of the 2nd Annual Conference of Nigerian Institute of Food Science and Technology, 21: 61-70 (1981).






