Himalayan snow chemistry: Chemical composition of fresh snow samples from Kashmir valley
F.A. Lone1 and M.A. Khan2 *
1
Division of Environmental Sciences,
Shalimar Campus,
S.K.University of Agricultural Sciences and Technology of Kashmir,
Srinagar,
191121
Kashmir
India
2
GPO Box 726,
Srinagar,
190001
Kashmir
India
DOI: http://dx.doi.org/10.12944/CWE.2.1.03
The present paper deals with variability in snow chemistry of various sites spread in six districts of the Kashmir Himalayan valley, experiencing frequent snow fall during winter ( Dec.- Feb). Chemical composition of fresh snow samples shows wide changes in pH (5.8–7.3), dissolved oxygen content (22-26 mg L-1), and total alkalinity (2-3 mg L-1). The levels ( mg L-1) of calcium (13.3– 28 ), magnesium (8-12 ), chloride (15-30), sulphate (2-8), silicate (1-4) and nitrate (1.9-3.9) differed considerably. The study makes a strong plea for in-depth investigations of snow chemistry of little-explored Himalayan region, useful for assessing the impact of environmental pollution.
Copy the following to cite this article:
Lone F.A, Khan M.A. Himalayan snow chemistry: Chemical composition of fresh snow samples from Kashmir valley. Curr World Environ 2007;2(1):17-20 DOI:http://dx.doi.org/10.12944/CWE.2.1.03
Copy the following to cite this URL:
Lone F.A, Khan M.A. Himalayan snow chemistry: Chemical composition of fresh snow samples from Kashmir valley. Curr World Environ 2007;2(1):17-20. Available from: http://www.cwejournal.org/?p=615
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2007-02-06 |
|---|---|
| Accepted: | 2007-04-23 |
Introduction
Various attempts have been made (,Reynolds, 1983, Jenkins et al 1987; Gunz and Hoffman, 1990; Marinoni et al, 2001; Toom-Sauntry and Barrie, 2002; Rohrbough et al 2003; Walker et al 2003 Kang et al 2005) to document the chemical composition of snow in different parts of the world. However, except for sporadic studies (,Naik et al 1995; Sarfaraz et al 2001; Lone and Khan 2007) research on snow chemistry of mountain- clad Kashmir Himalayan valley has been ignored. In order to remedy this deficiency in environmental chemistry of the region, an attempt has been made to analyze the hydrochemistry of fresh snow samples collected from various sites spread in six districts of Kashmir valley.
Material and Methods
Sampling Sites
The Kashmir valley of Jammu and Kashmir State is situated between 320 17’ - 370 15’ N and 720 40’ - 800 30’ E and occupies a strategic position in the north of India. Srinagar city, the summer capital of J&K State is located at an average altitude of 1500-1600 m asl and its atmosphere receives inputs mainly from automobiles and small - scale industries.The three sampling sites viz Lal Chowk, HMT area and Shalimar were selected in the district Srinagar. Whereas, the atmosphere of former two sites remains polluted with automobile emissions and the operation of small-scale industrial units, the other one is relatively a non-polluted area. The other sampling sites viz; Rafiabad, Dragmulla, Tral, Magam and Anantnag spread in various parts of Kashmir valley are located in district Baramulla, Kupwatra, Pulwama, Budgam and Anantnag respectively.
The winter season in Kashmir valley extends from Dec- Feb and is marked by cloudy and cold weather. On an average 7-8 winter disturbances pass east- wards in winter months affecting the northern most part of India. These disturbances are accompanied by continuous rain and appreciably low temperature, helping the formation of snow and ice in these regions (Naik et al, 1995) and approximately 50% of these affect the region covering Jammu and Kashmir, Himachal Pradesh, Haryana and the hills of western Uttar Pradesh and Uttranchal. The snowfall recorded at Agrometerological Observatory Shalimar (SKUAST- K) Kashmir during the winter of December, 2004 and Feb. 2005 is given in Table 1.
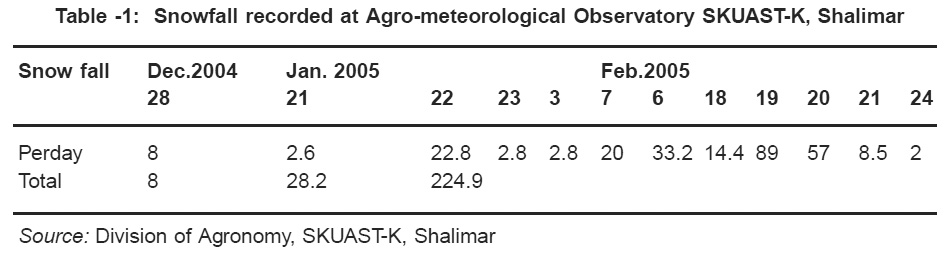 |
Table 1: Snowfall recorded at Agro-meteorological Observatory SKUAST-K, Shalimar Click here to view table |
Sampling Procedure and Analytical Methods
The fresh snow samples were collected from various sampling sites on the dates shown in Table 2. The snow samples were collected with a glass cylinder 40 cm long and 5 cm diameter.
Each sample was melted in the plastic tub covered with a glass sheet pre-cleaned with deionized water and allowed to reach ambient room temperature. After melting, the snow water was transferred into a 1 liter capacity polythene bottles which was sealed and returned to the laboratory for analysis.
The pH of the snow- melt was measured immediately by using electronic digital pH meter (Systronics 327). Methods recommended by Mackereth (1963) and the American Public Health Association (1992) were used for various physical and chemical variables. The Cl content was determined tritrimatrically against AgNO3 and total alkalinity was estimated against standard solution of H2SO. Dissolved oxygen (DO) was determined by Winkler’s reagent and titrated against standard solution of Na2S203,. Nitrate-nitrogen was estimated spectrophotometrically following phenol- disulphonic acid method, while sulphate was determined by the turbidimetric method. Silicate content was also determined colorimetrically by using acid-molybdate solution. Analysis of cations (Ca and Mg) was done titrametrically using Na2EDTA (disodium dihydrogen ethylene diamine-tetraacetate).
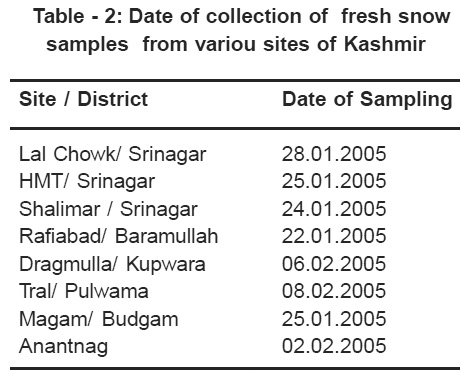 |
Table 2: Date of collection of fresh snow samples from variou sites of Kashmir Click here to view table |
Results and Discussion
The average concentration of pH and dissolved oxygen content, and major ionic forms of the snow samples collected from different sites of Kashmir valley are given in Table 3. The data indicate that the pH of the snow samples was the lowest (5.84) in HMT area of Srinagar city ,and for other sites pH values ranged from 6.2 (Anantnag) to 7.31 (Shalimar). The reference level commonly used to compare acid precipitation to natural precipitation is pH 5.6, the pH that results from the equilibration of CO2 with precipitation. This reference level has been chosen because of the ubiquity of CO2 in the global atmosphere and because of the absence of data on other acids or basics (Naik et al 1995).However, Galloway et al (1982) believe that there is no single natural pH of precipitation applicable to the whole globe, but rather several natural values, each unique to a region, the size of a continent or an ocean. The lowest values for pH in the snow samples of the HMT area could be attributed to the presence of higher concentration of species like NO3 and SO4 in the atmosphere of this region due to the operation of small- scale industrial units involving burning of coal, oil and timber.
The chloride content and total alkalinity in the snow samples ranged from 15-28 and 2.-3 mg L-1 respectively in different zones of Kashmir valley and suggest a strong influence of alkaline dust particles which might have originated from Indian plains and arid zones in the west and have been transported to the region by south westerly, winds (Naik et al 1995). The data also indicate that dissolved oxygen content in the snow samples collected from different sites of Kashmir valley ranged from 22-24 mg L-1 and suggests a fairly good concentration of O2 in the atmosphere.
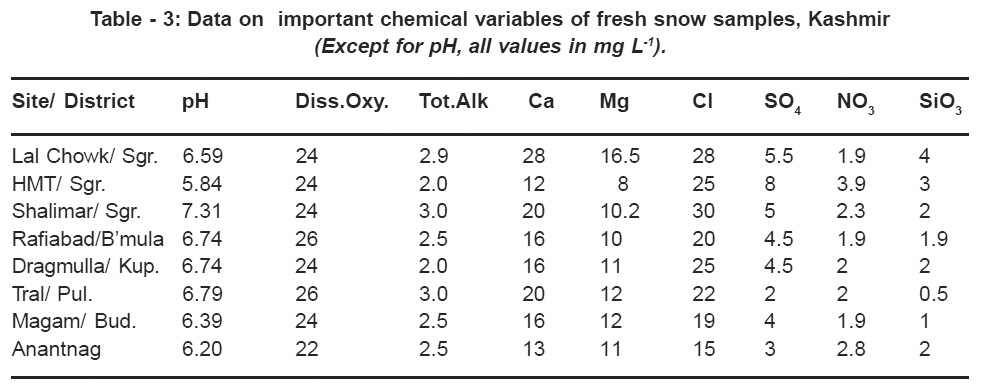 |
Table 3: Data on important chemical variables of fresh snow samples, Kashmir (Except for pH, all values in mg L-1). Click here to view table |
High concentration of NO3 (3.9 mg L-1) and SO4 (8 mg L-1) were recorded in the HMT area compared to other sites. Since SO2 and NO2 are mainly derived from anthropogenic sources, the high levels of these species in the snow samples of HMT area are likely to be due to industrial activity in the area. The other sites (e.g. Lal Chowk ) has significantly higher traffic-flow with appreciably higher NOx concentration in the atmosphere. However, during night time, the traffic usually remains off the road and consequently snow recorded lesser concentration of these species as the snowfall was recorded during the night hours. Under natural conditions, nitrate particles of submicron size in the atmosphere may be formed by gas to particle conversion process (Cox, 1974). Under normal atmospheric conditions, HNO3 can initially form Aitken nuclei which will grow rapidly into a submicron sizes and react with NH4 to NH4 NO3. This reaction is reversible and temperature dependent (Wolff, 1984). The formation of NH4NO3 in tropical countries is rare due to persistence of higher temperature. However, HNO3 can react with soil derived or some industrial particulates and become incorporated in snow as the coarse aerosol mode (Wolff, 1984). It is also clear from the data that the other sites( viz. Shalimar, Rafiabad, Tral, Magam and Anantnag) have almost negligible traffic-flow and consequently low concentrations of the NO3 and SO4 were observed during the present study.
The data also reveal that HMT site recorded low content of Ca2 (12 mg L-1)and Mg (8 mg L-1) . However, highest values were observed in Lal Chowk area ( Ca=28.05 and Mg= 16.5 mg L-1). For other sites (Table 3), the values differed markedly; Anantnag ( Ca=13 mg L-1) and Shalimar/ Tral (Ca=20 mg L-1). The Magnesium content fluctuated narrowly between 10-12 mg L-1 for the sites. The influence of alkaline components on the pH of the rain, cloud and fog water in India has been reported earlier (Khemani et al 1987 ). The higher concentration of alkaline components (Ca and Mg) are responsible for the higher pH of precipitation in India and the alkaline pH in the snow samples collected from Lal Chowk, Shalimar, Rafiabad, Dragmulla, Tral, Magam and
Anantnag is consistent with these observations. The presence of appreciably higher concentration of Ca has also been reported in the snow samples of Mt. Everest by Marinoni et al (2001). Other related studies have also shown higher ionic concentration of Ca in the premonsoon snow and have opined that it may be as a result of dust deposition during the peak dust storm activity mainly in April and May over Asia (Parrington et al, 1983). On the other hand, relatively low concentration of Ca and Mg in the snow samples collected from HMT area may also be one of the factors responsible for its low pH value.
Conclusion
Analytical research on ionic composition of fresh snow is a useful tool for assessing the environmental impact of air pollution sources as well as the transport and deposition of various air pollutants. The study has indicated the presence of highly appreciable concentration of anions (Cl) total alkalinity, MgSO4 and SiO3 as well as cations (Ca and Mg) in the fresh snow samples collected from different areas of Kashmir valley., The presence of these ions seems directly related to site-specific environmental conditions of the Himalayan region.
References
- American Public Health Association. Standard Method for the Examination of Water, Sewage, and Industrial Wastes.A.P.H.A. Inc.New York 626. pp. (1992).
- Cox ,R.A.. Particle formation from homogenous reactions of sulphur dioxide and nitrogen dioxide. Tellus, (1974) 26, 235-40.
- Galloway,J.N., Linkens G.E., Keena, W.C. and Miller J.M..The composition of precipitation in remote areas of world.J.Geophys Res. (1982) 87, 8771-86.
- Gunz ,D.W and Hoffmann.M .R.. Field investigations on the snow chemistry in central and southern California-1. Inorganic ions and hydrogen peroxide . Atmos. Environ. (1990) 24A 1661-72.
- Jenkins, M.D, Drever, J,J., Reider, R.G.and Buchanan, T. Chemical Composition of fresh snow on Mount Everest . J.Geophys. Res. (1987) 92, 10999-11002.
- Kang, S., Mayewski, P.A., Qin. D., Sneed, S A ., Ren, J and Zhang, D . Seasonal differences in snow chemistry from the vicinity of Mt. Everest Central Himalaya Atmospheric Environment. (2004) 38, 2819- 29.
- Khemani,L . T. ,Momin,G . A . ,Naik,M . S. Rao,P.S.P , Safai ,P.D.and Murty, A.S.R. Influence of alkaline particulates on pH of cloud and rain water in India Atmos. Environ. (1987) 7,162 – 68
- pp 245-56.(2007 Ed. M.A.Khan). APH Publ.Corp. New Delhi,Management and Sustainable Agriculture ( Kashmir Himalaya. In: Waters ResourcesLone,F.A. and Khan,M.A. Muddy precipitation phenomenon spells environmental pollution in
- Mackereth, F.J.H. Water analysis for limnologist. Freshwat. Biol. Assoc. (1963) 40, 1-70.
- Marinoni, A., Polesello, S ; Smiraglia, C and Valsecchi, S. Chemical composition of fresh snow samples from the southern slope of Mt.Everest region (Khumbu- Himal region ,Nepal). Atmos. Environ. 3183-90.
- Naik, M .S., Khemani, L. ., Momin, G. A., Rao, P.S.P., Safai, P.D. and Pillai A.G. Chemical composition of fresh snow from Gulmarg, North India. Environ. Pollut. (1995) 87, 167-71.
- Parrington. J.R., Zoller,W. H. and Aras, N.K . Asian dust: seasonal transport to the Hawaiin islands. Science (1983) 220,195- 97.
- Reynolds, B. The chemical composition of snow at a rural upland site in Mid-Wales. Atmos Environ (1983) 17,1848-51.
-
Rohrbough ,J.A., Davis ,D.R and Bales ,R.C. Spatial variability of snow chemistry in an alpine snow peak, Southern Wyoming. Wat. Resour. Res. (2003) 39,1190
-
Sarfaraz ,A., Hasnain ,S.I. and Ahmad, S. Snow and stream water chemistry of the Ganga head water basin, Garhwal Himalaya, India. Hydrol. Sci. J. ( 2001) 46:1, 103-11.
-
Toom-Sauntry, D and and Barrie ,L.A.. Chemical composition of snowfall in the high Arctic:1990-1994; Atmos. Environ. (2002) 2683-93.
-
Walker,T.R., Young, S.D. ., Crittenden, P.D. and Zhang, H Anthropogenic metal enrichment of snow and soil in north- eastern European Russia. Environ. Pollut. (2003) 121, 11-2.
-
Wolff, G. T. On the nature of nitrate in coarse continental aerosol. Atmos. Environ. (1984) 18, 977-81.






