A study of iron and some traces elements in ground water of Bhander and Seondha block (Datia, M.P.)
Naveen Kumar Singh1 * and D.S. Kadam2
1
Chemical Research Laboratory, Department of Chemistry,
SMS Government Science College,
Gwalior,
India
2
Government Chemical Laboratary Division,
Ground water survey,
Gwalior,
India
DOI: http://dx.doi.org/10.12944/CWE.2.1.11
Ninety six (96) ground water samples collected from Bhander and Seondha block (Datia District) were analyzed for Iron., Copper, Manganese, Zinc, and Lithium. Average was found for Iron., Copper, Manganese, Zinc, and Lithium were 0.257,0.019,0.037,0.014,0.011 ppm respectively.
Copy the following to cite this article:
Singh N.K, Kadam D.S. A study of iron and some traces elements in ground water of Bhander and Seondha block (Datia, M.P.). Curr World Environ 2007;2(1):57-60 DOI:http://dx.doi.org/10.12944/CWE.2.1.11
Copy the following to cite this URL:
Singh N.K, Kadam D.S. A study of iron and some traces elements in ground water of Bhander and Seondha block (Datia, M.P.). Curr World Environ 2007;2(1):57-60. Available from: http://www.cwejournal.org/?p=631
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2007-04-03 |
|---|---|
| Accepted: | 2007-04-30 |
Introduction
Ground water is one of the best source for public water supplies in many parts of the world.The physiological importance of trace elements in agriculture is well known while some of the elements like iron manganese, zinc and copper are considered to be essential micro nutrients the elements like lithium and boron have been found to be toxic to plants even in small quantities beyond certain limits Gupta et al(1972) reported lithium in saline ground waters of Mathura district (U.P.) and also examined the lithium tolerance or different crops at germination and seedling stage though Gupta (1974) observed the critical limits of lithium as 5ppm to be toxic to wheat and barley,Binghan et al (1964) reported the concentration limit of even 0.05 to 0.1 ppm as toxic to citrus crops. In the present investigation an attempt has been made to determine the concentration of some trace element viz. iron, zinc, copper, manganese and lithium.
Material and Methods
96 samples water from different parts of the Bhander and Seondha Block (Datia district) were collected and analyzed for all major cations, anions, pH and E.C. the determination of trace elements was carried out by atomic absorption spectrophotometer. The water have been classified into 4 groups on the basis of electrical conductivity (micromhos per cm at 25 0C ) each class average, minimum and maximum concentration of each elements was determined. EC values of the ground water samples under investigation were measured using systronic EC meter.
Results and Discussion
Iron is an essential element in the metabolism of animal and plants .If present in water in excessive amounts .However ,It forms red oxyhydroxide precipitates that stain laundry and plumbing fixtures and there fore is an objectionable
impurity in domestic and industrial water supplies for this reason ,iron determinations are commonly included in chemical analyses of water. A recommonmede water supplies is 0.3mg/L (NASNAE, 1972). The maximum permissible limit for Iron in Drinking water, prescribed by W.H.O. is 0.3-1.0 ppm, and I.S.I. has maximum limit 0.3 ppm. High in take may cause bacterial activity (redrot disease). Iron in ground water samples were varied from 0.000 ppm to 0.64 ppm. Modern industrial civilization uses copper extensively and many of theses uses result in its dispersal in the environment. Copper is an essential element in plant and animal metabolism.
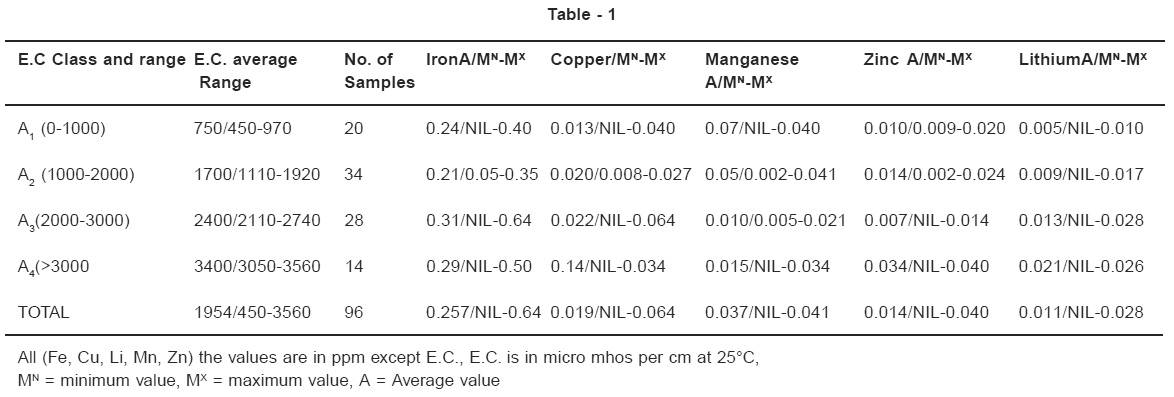 |
Table - 1 Click here to view table |
The U.S. environmental protection Agency (1976b) mandatory drinking water standard do not include a value for copper. An upper limit of 1mg/L of copper in public water supplies was suggested in Water quality criteria,1972 (NAS-NAE,1972), which also stated that this limit was based on the taste threshold for this element. The same report suggested an upper limit of 0.5mg/L in water to be used by livestock, and 0.20mg/L for continuous use in irrigation of crops. Toxicity for fish varies with species and major ion concentrations of the water , but the NAS-NAE report implies that concentrations greater than a few hundredth of a milligram per liter are potentially harmful for many species. The maximum permissible limit for copper in drinking water, prescribed by W.H.O. is 1.0mg/L and I.S.I..has maximum limit 0.5 -1.5 ppm .Deficiency of copper may cause nutritional anaemia in infants.
The value of copper in ground water samples were varied from0.000 ppm to 0.064 ppm. Manganese is an undesirable impurity in water supplies mainly owing to a tendency to deposit black oxide stains . The recommended upper limit for manganese in public water supplies in the united states is 0.05 mg/L(NAS NAE, 1972) no mandatory limit is specified for this element by the U.S. Environmental protection agency. It is an essential element for both plant and animal life forms The maximum permissible limit for Manganese in drinking water, prescribed by W.H.O. is 0.1 ppm and I.S.I.has maximum limit 0.1- 0.5 ppm. Its deficiency may cause in hibit growth disrupt to the nervous system and interfere with reproductive system. High in take may cause manganisum disease .The value of manganese in ground water samples were varied from 0.000ppm to 0.0.041ppmZinc is essential in plant and animal metabolism but water is not a significant source of the elements in a dietary sense. Water quality standard suggested by water quality criteria 1972(NAS-NAE1972) gave an upper limit of 5mg/L for zinc, be causeabove that limit a significant number of people can detect zinc by taste. No health effects were considered likely .Zinc is an undesirable contaminant for some species of aquatic life at much lower concentrations (NAS-NAE 1972p182) ,but the amount that can be tolerated is also a function of other properties of the solution. The maximum permissible limit for Zinc in drinking water, prescribed by W.H.O. is 5.0 ppm and I.S.I.has maximum limit 5-15 ppm. High in take may cause Diarrhoea, Weight loss, hair loss. Zinc Values varied for ground water samples from 0.000ppm to 0.040 ppmLithium can be toxic to plants according to Bradford (1963) citrus trees may be damaged by irrigation water containing 60 to 100 micro gram/ Liter .Lithium in ground water samples were varied from 0.000ppm to 0.028 ppm.Among the trace elements iron is most abundant in ground water and is also present in most of the water samples.The maximum concentration of Iron,Copper, Manganese, Zinc, and Lithium has been observed as 0.64,0.064,0.041,0.040,0.028 ppm respectively.
Acknowledgements
The authors are thankful to Dr. K.P.S Chauhan (Principal)for his invaluable guidance and help in determination of the trace elements.
References
- Rainwater F.H. and Thatcher .L.L., Method for collection and analysis of water samples USGS paper 1454 Washington (1970).
- Singh H.G. &Sharma R.P. ,Response of tobacco to brackish water, Indian J. agric.sci., (1971) 41, 697-699.
- Bingham F.T. ,page A.L. and Bardford G.R., Tolerance of plants to lithium, Soil Sci. (1964) 64, 4-8.
- Gupta I.C. , Note on Lithium in saline waters, Indian J. agric. Sci. (1972) 42, 650-651.
- Gupta I.C., Lithium tolerance of wheat, barley, rice and gram at germination and seeding stage. Indian J. agric Res., (1974) 8, 103-107.
- Silvey W.D. ,Occurrence of selected minor elements in water California U.S. Geological survey water –supply paper (1967) 1535-L, 25p.
- Paliwal K.V., Iron contents in ground water of Assam ,A survey Report on Tube wells, submitted to ICAR New Delhi (1976).
- Gibbs C., Characterization and application of ferrozine iron reagent as ferrous iron indicator, Anal. Chem., (1976) 48, 1197.
- Morris R.L., Determination of iron in water in the presence of heavy metals, Anal.Chem. (1952) 24,1376.
- W.H.O. Geneva ,guidelines for Drinking water quality, Vol I&II, (1987).
- Bradford G.R., Lithium survey of California s water resources: Soil Science, (1963) 96, 77-81.
- APHA(American public health association),American water works association and water pollution control federation, standard methods of examination of waster and waster water , 19th Edition ,New york,U.S.A.,(1995).
- Pawar C.T. and Joshi M.V., Impact of Urbanization and Industrialization on water Quality, Nat. Environ. Poll. Tech., (2002) 1(4), 351.
- National Academy of Sciences- National Academy of Engineering (NAS-NAE) ,Water Quality criteria,1972:Washington,D.C. National Academy of Sciences, (1972) 594.
- Underwood E.J., Trace elements in human and animal nutrient ,3rd edn. New
- York Academic Press (1971).
- Chaney R.I. and Giordano P.M., Microelements as related to plant deficiencies and toxicities, Soil Sci, Soc. Amer Madison Wisconsin, (1977) 233-280.
- Olaniya M.S. Et al Heavy metal pollution of agricultural soil and vegetation Due to application of municipal solid waste-A case study, Ind. J. Env. Health, (1998) 40, 160-168.
- IS 2490 ,Tolerance limits for industrial effluents prescribed by Indian Standard Institution (part-I) ,(1981).
- I.S. 105000,Indian standard Drinking water specification. Bureau of Indian Standards, New Delhi, 5, (1991).






