Inhibition of acidic corrosion of iron by some Carrageenan compounds
I. Zaafarany1 *
1
Department of Chemistry, Faculty of Applied Sciences,
Umm Al- Qura University,
P.O.Box - 118,
Makkah Al Mukaramha,
Saudia Arabia
DOI: http://dx.doi.org/10.12944/CWE.1.2.02
The corrosion inhibition of some sulfated water soluble natural polymer (carrageenan) compounds on the corrosion of iron in 1M HCl was studied using weight loss and galvanostatic polarization measurements. The percentage inhibition efficiency was found to increase with increasing concentration of inhibitor and with decreasing temperature. The inhibitive action of these compounds was discussed in terms of blocking the electrode surface by adsorption of the molecules. The adsorption process follows a Langmuir adsorption isotherm. The effect of temperature on the rate of corrosion in the absence and presence of these compounds was also, studied. Some activation thermodynamic parameters were calculated.
Copy the following to cite this article:
Zaafarany I. Inhibition of acidic corrosion of iron by some Carrageenan compounds. Curr World Environ 2006;1(2):101-108 DOI:http://dx.doi.org/10.12944/CWE.1.2.02
Copy the following to cite this URL:
Zaafarany I. Inhibition of acidic corrosion of iron by some Carrageenan compounds. Curr World Environ 2006;1(2):101-108. Available from: http://www.cwejournal.org/?p=595
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2006-10-05 |
|---|---|
| Accepted: | 2006-11-25 |
Introduction
Iron is the most used of all the metals in several fields of industry. Iron and similar metals can be exposed to corrosion in connection with environmental conditions. The corrosion rate varies subject to structure of ions and molecules, kind of ion, concentration of ion, kind of solution and kind of materials.1,2 Especially in environments containing Cl- ions, corrosion of metals is unavoidable, as Cl- ion is an active one, it forces oxide formation on metal surface. This increases the corrosion rate of metal.2
Organic additives are commonly used to reduce the corrosion of iron in acidic medium.3-8 The inhibition efficiency of such compounds may function by adsorption on the iron surface, the adsorption process depends mainly on certain physico-chemical properties of the molecules such as, functional groups, steric factors, aromaticity, electron density at the donor atoms, π- orbital character of donating electron9,10 and also on the electronic structure of the molecules. The inhibition efficiency increases with increasing the number of atomic rings.11,12
The aim of the present work is to study the inhibition action of some naturally occurring compounds known as carrageenans which produced from certain species of sea weeds to inhibit the corrosion of iron electrode in 1M HCl solution using weight loss and galvanostatic polarization technique. The effect of temperature on the dissolution of iron electrode in 1M HCl containing 500 ppm of the inhibitor used was also studied and some thermodynamic parameters were computed.
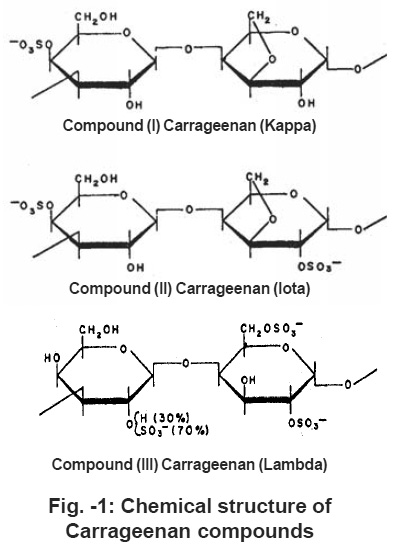 |
Figure -1: Chemical structure of Carrageenan compounds Click here to view figure |
Experimental
The test electrode was made of iron obtained from Saudi iron and steel company and having the following chemical compositions (%) (C, 0.052, Mn 0.189, S 0.011, P 0.008, Si 0.011, Al 0.039, N 0.001, Cr 0.0128, Cu 0.04, Mo 0.024, Ni 0.0293 and remainder is Fe).
Iron sheets with dimension 2x2x0.1 cm were used for weight loss measurements. For galvanostatic polarization technique a cylindrical rod embedded in araldite with exposed surface area of 0.5 cm2 was employed. Prior to each experiment, the surface of iron specimens were mechanically polished with different grades of emery paper, degreased with acetone and rinsed by distilled water.
For weight loss measurements, the cleaned Fe sheet was weighed before and after immersion in 50 ml of the test solution for a period of time up to 3 hours. The average weight loss for each two identical experiments was taken and expressed in mg cm-2, the temperature was adjusted to 25 ± 0.1 °C using air thermostat.
| Figure - 2: Weight loss-time curves for thecorrosion of iron electrode in 1M HCl inabsence and presence of differentconcentrations of compound III. Click here to view figure |
The loss in weight of metal in the corrosive solution
W = WB - WA ...(1)
where
W = Loss in mass of metal in the corrosive solution. WB = Weight of metal before exposure to the corrosive solution.
WA = Weight of metal after exposure to the corrosive solution.
The corrosion rate Rcorr was calculated from the following equation[13]:
...(2)
where,
S is the surface area (cm2). t is the time (min), after 150 min.
The percentage inhibition efficiency (%I.E) of the selected carrageenans compounds were calculated using the following equation
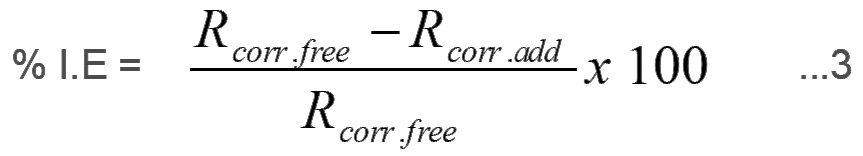
where,
Rcorr.add and Rcorr.free are the corrosion rates of iron in the absence and presence of the carrageenan compounds, respectively.
For galvanostatic polarization measurements were carried out using Mensberger potentiostat galvanostatic PS6 with controlling measurements of potential and current density. Three compartments electrode (SCE) and a platinum foil auxiliary electrode was used.
The percentage inhibition efficiency (%I.E) of the selected carrageenan compounds were calculated using the following equation
...(4)
where,
Ifree and Iadd are the corrosion current densities in the absence and presence of the inhibitors, respectively.
Results and Discussion
Weight loss measurements
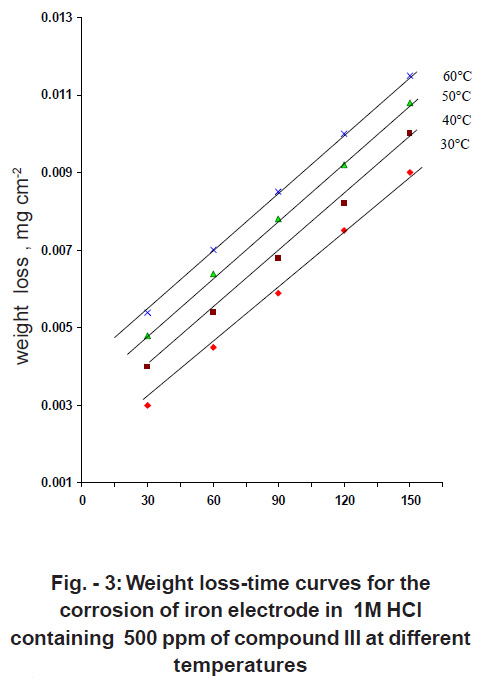 |
Figure - 3: Weight loss-time curves for thecorrosion of iron electrode in 1M HClcontaining 500 ppm of compound III at differenttemperatures Click here to view figure |
Fig. 2 represents the relation between time and weight losses of iron coupons in 1M HCl solution devoid of and containing different concentrations of compounds III as an example. Similar curves were also obtained for other two compounds (not shown). Inspection of this figures reveals that, as the concentration of these compounds increases, the loss in weight of iron compounds decreases. This reflects that, these compounds acts as an inhibitor. The linear variation of weight loss with time in uninhibited and inhibited 1M HCl solution indicates the absence of insoluble surface film during corrosion i.e. the inhibitor are first adsorbed on the metal surface and there after impede corrosion either by merely blocking the reaction sites (anodic and cathodic) or by uttering the mechanism of the anodic and cathodic processes.
| Figure - 4: Relation between log Rcorr and the reciprocal of the absolute temperature ofiron electrode in: a) 1M HCl, b) 1M HCl + 500 ppm of the studied compound1) compound I 2) compound II 3) compound III Click here to view figure |
The calculated values of inhibition efficiencies obtained from weight loss are listed in Table 1. It is obvious that the I.E increases with increasing the inhibitor concentrations, whereas decreases in the following order.
compound III > compound II > compound I
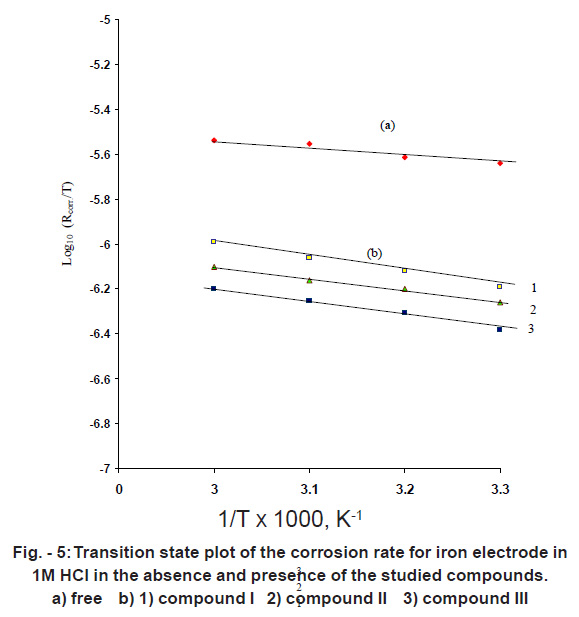 |
Figure - 5: Transition state plot of the corrosion rate for iron electrode in1M HCl in the absence and presence of the studied compounds. a) free b) 1) compound I 2) compound II 3) compound III Click here to view figure |
Effect of temperature
The effect of temperature on the corrosion rate of iron electrode in 1M HCl containing and devoid of 500 ppm of the studied these compounds was tested by weight loss measurements over a temperature range from 30-60°C.
Fig. 3 shows the weight loss-time curves of iron electrode in 1 M HCl containing 500 ppm of the compound III, at different temperatures. Similar curves were obtained for other two compounds I & II (not shown). It is clear that as the temperature increases the weight loss increases, the rate of corrosion increases and hence the inhibition efficiency decreases. This is due to desorption which is aided by increasing the temperature.
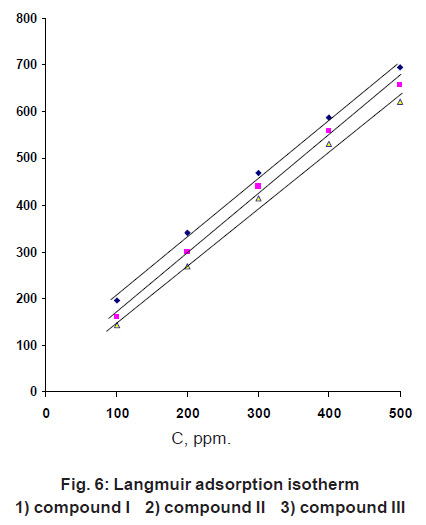 |
Figure 6: Langmuir adsorption isotherm 1)compound I 2) compound II 3) compound III Click here to view figure |
The activation energy (Ea) of the corrosion process was calculated using Arrhenius eq.14
...(5)
and the logarithmic form
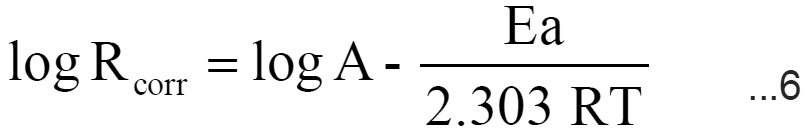
where,
Rcorr is the rate of corrosion from weight loss, A, is Arrhenius constant , R is the gas constant and T is the absolute temperature.
Fig. 4 represents Arrhenius plot (log Rcorr vs. I/T) for uninhibited and inhibited 1M HCl containing 500 ppm of the studied compounds. The values of Ea can be obtained from the slope of the straight lines and have been given in Table -2.
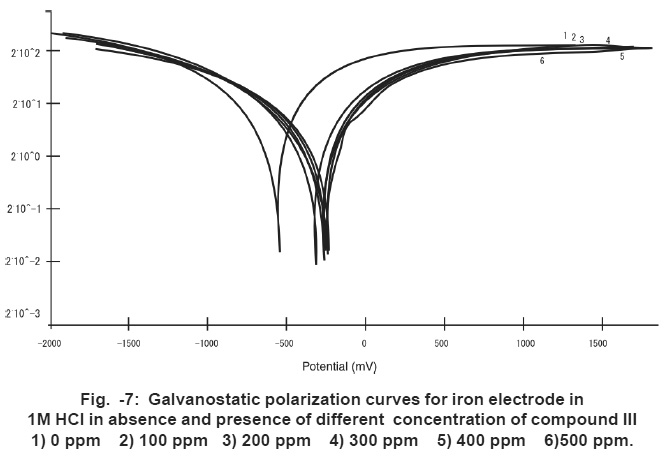 |
Figure -7: Galvanostatic polarization curves for iron electrode in1M HCl in absence and presence of different concentration of compound III 1) 0 ppm 2) 100 ppm 3) 200 ppm 4) 300 ppm 5) 400 ppm 6)500 ppm. Click here to view figure |
Inspection of Table -2 it is obvious that the values of Ea increases in the presence of the inhibitor. This is attributed to an appreciable decreases in the adsorption process of the inhibitors on the metal surface with increase of temperature and a corresponding increase in the reaction rate because of the greater area of the metal that is exposed to the acid.15
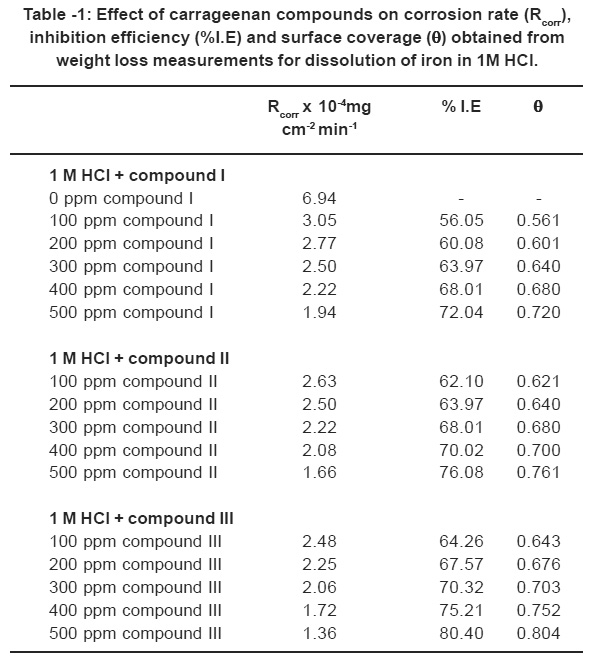 |
Table -1: Effect of carrageenan compounds on corrosion rate (Rcorr),inhibition efficiency (%I.E) and surface coverage (θθθθθ) obtained fromweight loss measurements for dissolution of iron in 1M HCl. Click here to view table |
The enthalpy change of activation ( H*) and the entropy change of activation ( S*) for dissolution of iron electrode in 1M HCl in presence and absence of 500 ppm of each used compound were obtained by applying the transition state equation.14
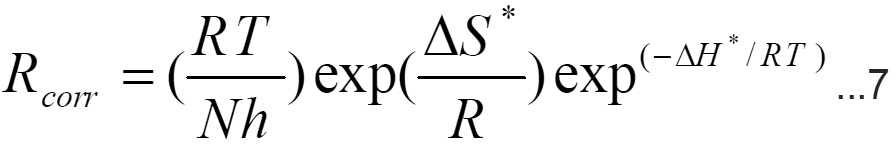
where
N is Avogadro number, h is Planck’s constant.
A plot of log (Rcorr/T) vs (1/T) (Fig.5) should give straight line with a slope of (- H*/2.303 R) and an intercept of [log (R/Nh)-( S*/2.303R)]. The values of DH*--- and DS* are listed in Table -2.
Inspection of Table it is clear that , the values of H* is positive. This reflect that the process of adsorption of the inhibitors on the iron surface is endothermic process. The values of S* is the presence and absence of the inhibitors is negative. This implies that the activated complex is the rate determining step and represents association rather than dissociation indicating that a decrease in disorder takes place on going from reactants to the activated complex.16
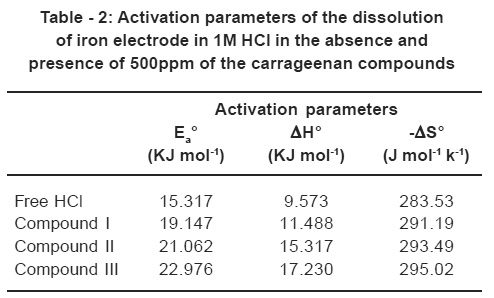 |
Table - 2: Activation parameters of the dissolutionof iron electrode in 1M HCl in the absence andpresence of 500ppm of the carrageenan compounds Click here to view table |
Adsorption isotherm
The values of surface coverage θ for different concentrations of the studied compounds (I-III) at 25°C have been used to explain the best isotherm to determine the adsorption process. The adsorption of organic adsorbate on the surface of iron electrode is regarded as substitutional adsorption process between the organic compound in the aqueous phase (Orgaq) and the water molecule adsorbed on the electrode surface (H2O)ad.17
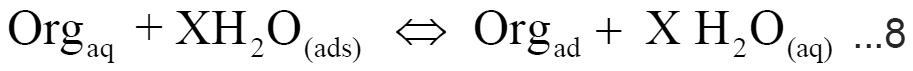
where,
X is the size ratio, that is the number of water molecules replaced by one organic molecules. Attempts were made to fit è values to various isotherm including Furmkin, Temkin, Freundilch and Langmuir isotherm. Plotting C/θ against C gave a straight line with unit slope value (Fig. 6) indicating the adsorption of carrageenan compounds on iron surface follows Langmuir adsorption isotherm.
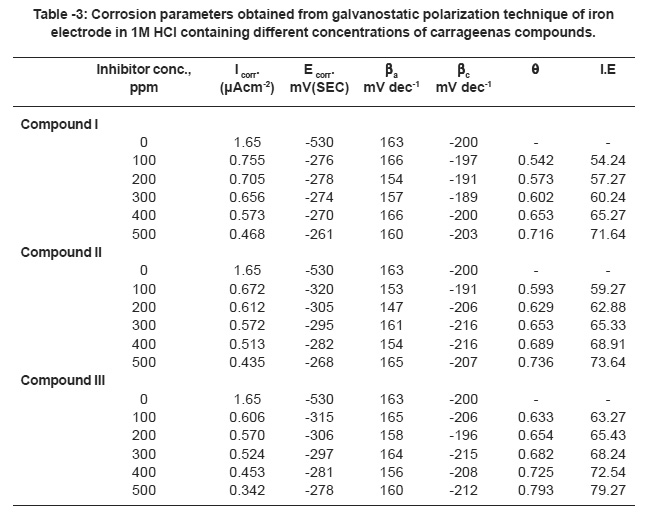 |
Table -3: Corrosion parameters obtained from galvanostatic polarization technique of ironelectrode in 1M HCl containing different concentrations of carrageenas compounds. Click here to view table |
From these results one can postulates that there is no interaction between the adsorbed species.
Galvanostatic Polarization
The effect of carrageenan compounds on the anodic and cathodic polarization curves of iron electrode in 1 M HCl was studied. Fig. 7 shows the effect of increasing concentrations of compound III as an example. However, similar curves were obtained for the other two compounds (not shown).
Inspection of Fig. 7 reveals that, the presence of increasing concentrations of carrageenan compound causes a marked decreases in corrosion rate i.e. shifts the anodic curves to more positive potentials and the cathodic curves to more negative potentials. This may be ascribed to the adsorption of the carrageenan compounds over the metal surface. The corrosion current density (Icorr) was determined from the intersection of the linear parts of the cathodic curves with the stationary corrosion potential (Ecorr).
The electrochemical parameters such as, corrosion potential (Ecorr), corrosion current density (Icorr) anodic and cathodic Tafel slopes (ba&bc) and inhibition efficiency (I.E) were calculated and given in Table 3. Inspection of the data cited in this Table reveals that , as the concentration of the additives increases, the values of ba & bc are approximately constant suggesting the inhibiting action of these compounds by adsorption at the metal surface according to blocking adsorption mechanism. The values of Ecorr is shifted to more positive potentials. This indicates that, this compounds are anodic inhibitors reducing the anodic reaction. The values of Icorr decreases and the values of I.E increases indicating the inhibiting effect of these compounds. The inhibition efficiencies of these compounds decreases in the following order:
compound III > compound II> compound I
which in consistent with that obtained from weight loss measurements.
Conclusions
- The corrosion of iron electrode in 1M HCl is inhibited by addition of some carrageenan compounds.
- The inhibition efficiency increases with increasing of the inhibitor concentrations and decreases with increasing of temperatures.
- The inhibitive effect of these compounds is attributed to the adsorption on the metal surface.
- The adsorption process follows Langmuir adsorption isotherm.
- The inhibition efficiency from weight loss measurements agrees with those obtained from polarization measurements.
- Investigated carrageenan compounds are anodic inhibitors reducing the anodic reaction.
References
- Zor, S., B. Yozici and M. Erbil: Corros. Sci., (2005) 47, 2700.
- Mu, G.N., T. P. Zhoo, M. Liu and T. Gu, Corrosion, (1996) 52, 853.
- Zor, S., B. Yazici and M. Erbil: Corros. Sci., (2005) 47, 2700,.
- Jeya Prabha, C., S. Sathiyanarayanan and G. Venkatachari: Electrochim Acta, (2006) 51 (19), 4080.
- Chetuani, A., B-Hammouti, T. Benhadda and M. Daoudi: Appl. Surface Sci., (2005) 249 (1-4) 375,.
- Abd El-Maksoud, S.A., Corros. Sci., (2002) 44, 803.
- Khaled, K.F.: Appl. Surf. Sci., (2004) 230, 307.
- Abdel-Rehim, S. S., K. F.Khaled and N.S. Abd-El Shafi: Electochem Acta, (2006) 51(16), 3269.
- Khamis, E., Corrosion, (1990) 46 , 476.
- Schmitt, G. and Bedbur:Werkst, Korros., (1985) 36, 273.
- S. L. Granese: Corrosion, (1988) 44, 322.
- Granese, S.L., B. M. Rosales, C. Obiedo and J. O. Zerbino: Corros. Sci., (1992) 33, 1439.
- Bensajjay, F., S. Alehyen, M. El-A Chouri and S. Kertit: Anti. Corro. Methods and Materials., (2003) 50(6),402.
- Putilova, I., S. Balezin, I. N. Barannik, V.P. Bishop: Metall. Corros. Inhibitor, Pergamon, Oxford, (1960) 196.
- Putilova, I.N., S. Balezin, V.P., Barannik, T. ​​​​​​​Baba: Corros. Sci., (1962) 2, 22.
- Abd El- Rehim, S. S., S. A. Refaey, F. Taha, M. B. Sleh and R. A. Ahmed: J. Appl. Electrochem., (2001) 31, 429.
- Moretti, G., G. Quartarone, A. Tassan, A. Zingales, Werkst, Korros. (1994) 45, 641.






