Comparative study of different agro based adsorbents for the treatment of wastewater
Sohail Ayub1 * , S. Iqbal Ali2 and N. A. Khan3
1
Civil Engineering Section,
Aligarh Muslim University,
Aligarh,
India
2
Environmental Engineering and University Polytechnic,
Aligarh Muslim University,
Aligarh,
India
3
Civil Engineering Department,
Faculty of Engineering and Technology,
Aligarh Muslim University,
Aligarh,
India
DOI: http://dx.doi.org/10.12944/CWE.1.2.03
In the present study attempts have been made to remove hexavalent chromium from the wastewater by agro based waste materials. The removal efficiency of the Cr (VI) has been determined at different dosage, concentration of metal and pH. It was found that agricultural waste adsorbents are superior to activated carbon. Since they are relatively cheaper and locally available. The isotherm data obtained is more closely to Freundlich adsorption isotherm for coconut shell and neem bark. Sugar cane bagasse follows Langmuir adsorption isotherm. The exhaustive capacity of the sorbent is higher in case of column process than the batch process
Copy the following to cite this article:
Ayub S, Ali S.I, Khan N.A. Comparative study of different agro based adsorbents for the treatment of wastewater. Curr World Environ 2006;1(2):109-116 DOI:http://dx.doi.org/10.12944/CWE.1.2.03
Copy the following to cite this URL:
Ayub S, Ali S.I, Khan N.A. Comparative study of different agro based adsorbents for the treatment of wastewater. Curr World Environ 2006;1(2):109-116. Available from: http://www.cwejournal.org/?p=597
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2006-10-10 |
|---|---|
| Accepted: | 2006-11-25 |
Introduction
The continually increasing demand for water for beneficial purposes has forced man to assess and examine water reuse technologies more seriously than ever. Regardless of origin, industrial wastewater, after proper treatment, represents another ample and reusable water source. Water pollution due to toxic heavy metals has been a major cause of concern to scientists and engineers.
Several mishaps due to heavy metal contamination in aquatic environment increased the awareness about heavy metal toxicity. Public awareness for pollution caused by heavy metals is now worldwide. Among the many heavy metals, lead (Pb), mercury (Hg), cadmium (Cd), arsenic (As), chromium (Cr), zinc (Zn), and copper (Cu) are of most concern, although the last three metals are essential nutrients for animals and human. They find wide spread usage in industries. These metals enter the environment wherever they are produced, used or discarded. They are very toxic because as ions or in compound forms, they are soluble in water and may be readily absorbed into living organisms. After absorption, these metals can bind to vital cellular components such as structural proteins, enzymes, and nucleic acids, and interfere with their normal functioning. In humans, some of these metals even in small amounts, can cause severe physiological and health disorders.
The compound of chromium, especially hexavalent chromium, are known to be detrimental to human beings, plants, animals and aquatic life (Stomberg, 1984; Tondon, 1982). Over exposure of chrome workers to chromium dust and mists will cause irritation and corrosion of skin, respiratory track and probably lung carcinoma (Hayes, 1982). Ingestion may cause epigestric pain, nausea, vomiting, severe diarrhea and hemorrhage (Browing, 1969). On the other hand, trivalent chromium has been found to be an essential trace element in the human diet and deficiency in trivalent chromium has been linked with poor sugar metabolism (Katz, 1991).
Naturally occurring chromium is chiefly in the form of chromites (Cr2O3) or chrome-ore (FeO-Cr2O3). Chromium compounds are widely used in the electroplating of metal for corrosion resistance, plastic coating of surfaces for water and oil resistance, leather tanning and metal finishing, and in pigments and wood preservative etc. and consequently discharging hexavalent chromium bearing wastewater. Anthropogenic production of hexavalent chromium in smaller amounts is through drilling mud, rust and corrosion inhibitors, textiles and toner for copying machines (Sujatha, et al., 1995). Hence it is a challenging task for the industrialists and environmentalists to dispose off waters containing heavy metals safely and effectively.
There are many techniques for the treatment of chromium bearing effluents, some of which are well established that have been in practice for decades such as precipitation, co-precipitation (Patterson and Passino, 1987), and concentration. These processes simply remove chromium from wastewater by reduction (Shen and Wang, 1995), coagulation and filtration. Although these technologies are quite satisfactory in terms of purging chromium and other heavy metals from wastewater, they produce solid residues containing toxic compounds whose final disposal is generally by land filling. It involves high costs and has possibility of ground water contamination. From environmental point of view, removing pollutants from liquid wastewater does not solve problem but transfer it from one phase (usually liquid) to another phase (usually solid). There is possibility that the presence of organic ligands and / or acidic conditions in the environment increases Cr (III) mobility, and also MnO2, in the soil, could oxidize Cr (III) to more toxic and mobile Cr (VI) forms (Heary and Ray, 1987). Accordingly, the practice of land filling and land application of chromium contaminating sludge should be discouraged.
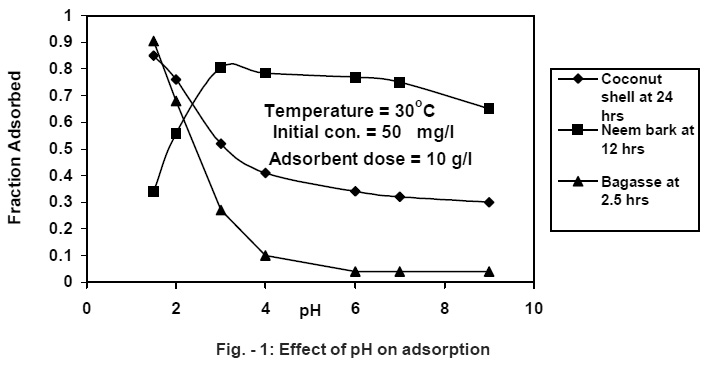 |
Figure - 1: Effect of pH on adsorption Click here to view figure |
In recent years, the use of adsorption techniques for the removal of heavy metals has received global attention (Raji, et al., 1998; Ajmal, et al., 1995, 2000; Huang, et al., 1975; Ayub,et.al.,1998,1999,2001,2002,2003). Chemical contaminants at low concentrations are difficult to remove from the wastewater. Chemical precipitation, reverse osmosis and other methods become inefficient when contaminants are present in trace concentrations. The process of adsorption is one of the few alternatives available for such situations (Huang and Morehart, 1991). Several researchers have been working on the heavy metals removal. However, most of them used commercially available activated carbon.
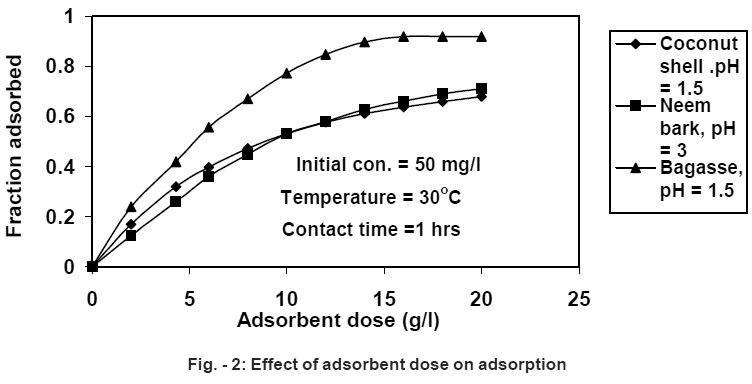 |
Figure - 2: Effect of adsorbent dose on adsorption Click here to view figure |
The high cost of activated carbon and its loss during the regeneration restricts its application. Thus in the present study commercial activated carbon was substituted with the unconventional, low cost and locally available agricultural waste adsorbents. India is an agricultural country and generates considerable amount of agricultural wastes such as sugar cane bagasse, coconut jute, nut shell, rice straw, rice husk, waste tea leaves, ground nut husk, crop wastes, peanut hulls, compost wastes etc. Recently few researchers have explored the possibility of using agricultural waste adsorbent for the Cr (VI) removal (Gang, et al., 1999; Deo, et al., 1992; Huang, et al., 1975; Periasamy et al., 1991; Ayub, et al., 1998, 1999, 2001, 2002; Drake, et al., 1996; Shukhla, and Sakhardane, 1991; Weber, 1996; Chand, et al., 1994; Siddiqui, et al., 1994; Camino, et al., 2000, Yaishya and Prasad, 1991).
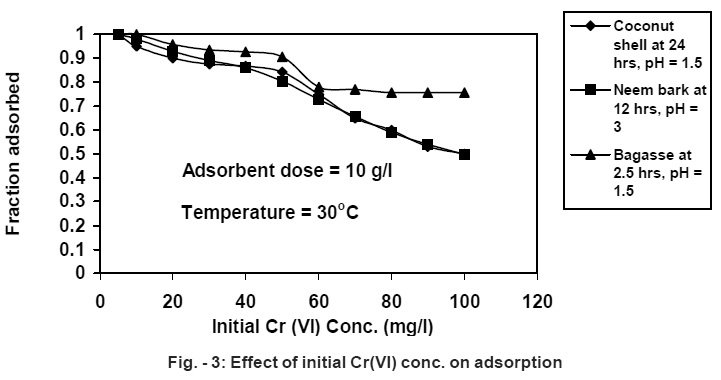 |
Figure - 3: Effect of initial Cr(VI) conc. on adsorption Click here to view figure |
The present study evaluates the heavy metal removal potential of agro-waste materials such as coconut shell, neem bark (Azadirachta indica) and raw sugar cane bagasse in the treatment of wastewaters. The effects of pH, contact time, adsorbent dose, concentration of metal, particle sizes and temperature were studied. The samples of the adsorbents were characterized before and after adsorption. The column studied were made to get the data to design a continues system and to compare it with the batch performance. Electron microscopic technique was used to characterize the surface of the adsorbent. Various adsorbents were used to determine the removal potentials of different agricultural wastes. Thermodynamic nature of the process was also studied.
Material and Methods
Collection of Samples and Site Description
Aligarh is a medium sized semi industrialized town located in northern India 135 Km South – East of New Delhi. The city is famous for the electroplating of locks and other building materials for the indigenous as well as export market. A number of samples from various parts of the town were analyzed to identify the contaminated surface waters. The samples of electroplating effluents were also collected from the discharge points of the factory / surroundings and its nearby and stored as per standard methods before analyzing the physico-chemical parameters. All the samples were tested for pH, chloride, alkalinity, suspended solids, hardness, total dissolved solids and heavy metals chromium contamination. The study reveals that most of the places the surface water is within the limit except a few places where the water was observed to be hard and contained chromium (VI) contamination. The details are given in Table 1. However, the chromium contamination in the surface water was found above the prescribed safe limit 0.1 mg/L.
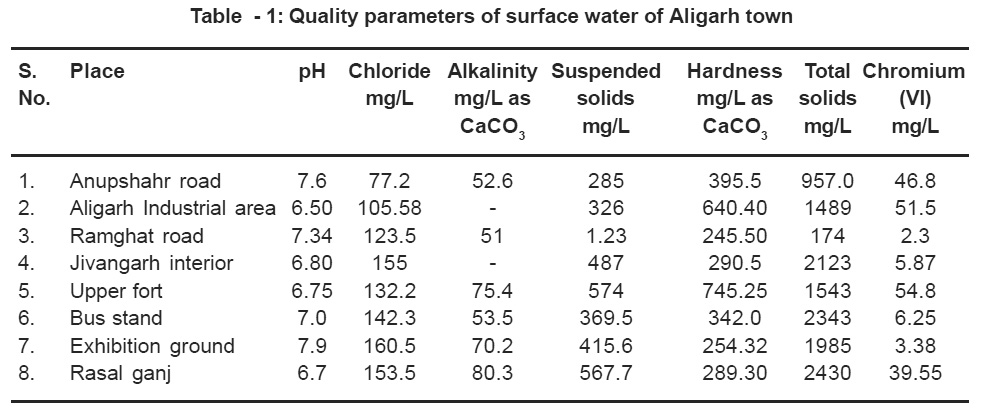 |
Table - 1: Quality parameters of surface water of Aligarh town Click here to view table |
Selection of adsorbents and Sample Preparation
In the present work various agro-based waste materials such as, coconut shell, neem bark (azadichta Indica) and raw bagasse have been used as adsorbents for the investigating the removal of Chromium (VI) from the wastewater using batch and column adsorption processes. These adsorbent are widely available in India. A considerable amount of different types of agricultural wastes are generated during the harvesting of various crops. These agricultural wastes if properly utilized can serve as a means of pollution control from various industrial units. Neem barks are widely available all over the India and coconut shell is a waste material easily available in the southern states. Chand, et al., (1994) also used raw bagasse for the treatment of hexavalent chromium without the characterization of adsorbent and carried out only effect of adsorbent dose, pH, Initial concentration, temperature and isotherm parameters in study. No other investigator has carried out adsorption on Neem bark and coconut shell for the removal of Cr (VI). As both materials are abundantly available throughout India, so it was considered to study the adsorption characteristics of both of them. Some investigator such as Arulnatham, et al., (1989) has studied removal of cadmium and lead on coconut shell but none of them have used Neem bark.
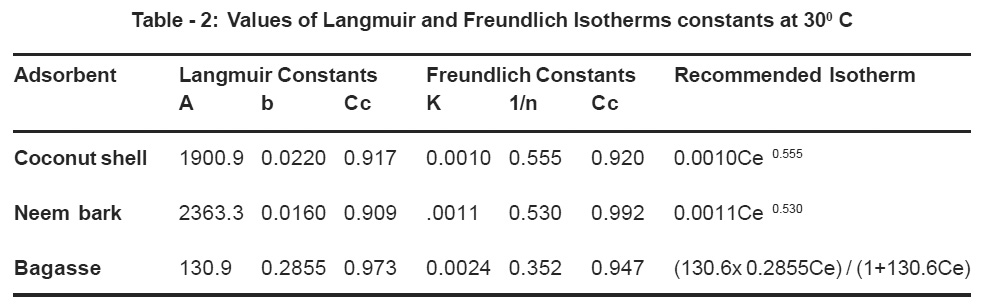 |
Table - 2: Values of Langmuir and Freundlich Isotherms constants at 300 C Click here to view table |
Adsorbents
The coconut shell, neem bark (azadichta Indica) and sugarcane bagasse was first dried at a temperature of 150° C for 5 hours. After grinding it was sieved to obtain average particle size of 225 mesh (Indian Standard Sieve). It was then washed several times with distilled water to remove lighter materials and other impurities. The adsorbent was dipped in 1N NaOH for a period of 10 hrs and washed several times with distilled water to remove the lignin content and then dried. The adsorbent was again washed separately with double distilled water two to three times and dipped into 0.1N H2SO4 for the period of 10 hrs to remove traces of alkalinity. The acid treated adsorbent is washed thoroughly with double distilled water. Thereafter the material was dried in sun and stored in a desiccator.
 |
Table - 3: Thermodynamic parameters at 300 C Click here to view table |
Experimental
In order to understand the adsorption behavior a number of batch studies were conducted according to the Standard Method to investigate the effect of adsorbent dose and contact time, pH, concentration of metal. For these studies, wastewater of various concentrations of Cr (VI) was prepared from the stock solution and kept separately in glass stoppered conical flasks. Then suitable doses of adsorbent were added to the wastewater in a conical flask of 250 ml. The system was equilibrated by shaking the contents of the flasks at room temperature on a mechanical shaker (Indian Scientific Instruments factory, Ambala Cant, India) so that adequate time of contact between adsorbent and the metal ion was maintained.
The suspension was filtered through Whatman (No. 1) filter paper and the filtrate was analyzed to evaluate the concentration of Cr (VI) metal in the treated wastewater by using atomic absorption spectrophotometer (GBC 902). Adsorption studies were made for various times. Ultimate saturation time was also determined for each dose. These studies were carried out at the room temperature which varied from 20-25o C in winter time to 30-38o C in summer time. In the elevated temperature studies the temperature was maintained by external heaters in closed chamber.
Batch Studies
In order to evaluate the effect of adsorbent dose and contact time, pH, concentration of metal, and temperature variation on the potential of agricultural waste adsorbents to remove Cr (VI) from the wastewater, batch experiments were designed.
Column Studies
Although batch laboratory adsorption studies provide useful information on the application of adsorption to the removal of waste constituents, column study provide the practical application in the waste treatment technology for the design of continuous adsorption columns. Column studies were conducted using a glass column (Internal diameter 1.0-cm). Adsorbent was suspended in distilled water for 15 minutes by shaking in a beaker at the speed of 150 rpm, and then transferred in to the glass column. This was done to disperse the particles properly and to avoid conglomeration. After this, particles were transferred in the column. The glass wool was kept at the bottom and top ends in order to avoid its loss with the liquid flow or floating. The flow rate was maintained at 1.0 l/d. Concentration in the influent and exit stream from the column were determined using atomic absorption spectrophotometer (GBC 902). The column experiments were conducted at the room temperature.
Results and Discussion
The experimental data obtained during the adsorption studies are being used to evaluate the effect of adsorbent dose and contact time, influence of pH, effects of various initial Cr (VI) concentrations. Adsorption isotherms are plotted to determine the feasibility of adsorbing system. Kinetic studies have been performed to understand the mechanism of adsorption. Thermodynamics parameters indicate the feasibility of the process. Column studies have been carried out to compare these results with the batch studies.
Comparison of Coconut Shell, Neem Bark and Sugar Cane Bgasse Adsorbents
The investigations reveal that agricultural waste adsorbents surfaces (Coconut shell, Neem bark and sugar cane bagasse) can be used as effective adsorbents for the removal of hexavalent chromium from electroplating and metal finishing wastewaters. Sorption of chromium is highly pH dependent as shown in Fig. -1 and the best results were obtained in the pH range 1.5-3.0. The reason for the better adsorption capacity observed at low pH values may be attributed to the large number of H+ ions present at these pH values, which in term neutralize the negatively charged hydroxyl group (-OH) on adsorbed surface thereby reducing hindrance to the diffusion of dichromate ions. At higher pH, the reduction in adsorption may be possible due to abundance of OH- ions causing increased hindrance to diffusion of positively charged dichromate ions. It is the common observation that the surface adsorbs anions favorably in low pH range due the presence of H+ ions (Gebehard and Coleman, 1974) whereas, the surface is active for the adsorption of cations at higher pH values due to the accumulation of OH-ions (Huang and stunm, 1973). Similar observations were recorded during the studies conducted on dihydric phenol removal on activated carbon by Mahesh, et al., (1999) and Sharma, et al., (1993) used sphagnum moss peat for the removal of chromium.
It is has been observed that the percentage removal increases with increasing adsorbent dose. 10 g/L dose of sugar cane bagasse is sufficient to adsorb more than 90 % Cr (VI) having 50 mg/L initial concentration within 1.0 hour. On further increasing the adsorbent dose to 16 g/L a 100 % removal efficiency were observed as shown in Fig. -2. Similarly, 10 g/L adsorbent dose of neem bark adsorbs more than 80% at pH 3.0 and coconut shell 85% at pH 1.5 respectively. Similar results were reported by (Ayub, et al., 2001, 2002; Rao, et al., 2002; Bansal and Sharma, 1992; Kim and Joltech, 1977; Singh, et al., 1992; Mall, 1992).
At the initial stage, Cr (VI) removal is very fast and the later stage of Cr (VI) removal is much slower. The extent of removal depends on the metal concentration, adsorbent dose, contact time, particle size and pH. Several investigators have reported that appreciable reduction in percent adsorption with increase in metal ion concentration (Panday, 1984; Kumar, 1987). Sugar cane bagasse is found to be superior to neem bark and coconut shell, as shown in Fig.-3. with respect to the removal efficiency of the above metal in 10 g/L adsorbent dose and having 50 mg/L initial concentration of the solution. The removal efficiency ranges from 80% - 90%.
For all the adsorbents, the Langmuir and Freundlich adsorption isotherms were plotted and the isotherms are given in the Table 2. It is observed that best fit straight line having correlation coefficient (Cc) value of 0.917 and 0.920 for coconut shell, 0.909 and 0.992 for Neem bark and 0.973 and 0.947 for bagasse. Hence it can be stated that the adsorption isotherm is represented more closely by Freundlich adsorption isotherm for coconut shell and neem bark, while Langmuir adsorption isotherm for bagasse.
The change in apparent enthalpy ( H), free energy ( G) and entropy ( S) of sorption were calculated using thermodynamic equations and values at 30 0C are listed in Table 3.0. The positive H values confirm the endothermic nature of the sorption process and suggested the possibility of strong binding between sorbate and sorbent for coconut shell and Neem bark. While negative H values confirm exothermic nature of adsorbent. Negative values of G indicate the process to be feasible and spontaneous. The negative values of free energy change for a system indicates spontaneity of adsorption process. Adsorption at a solid solution interface generally shows an increase in entropy. This indicates, a faster interaction during the forward adsorption. Association, fixation or immobilizations of adsorbate on the interface between two phases result in loss of the degree of freedom there by, showing a negative entropy effect. The positive entropy in case of coconut shell and Neem bark may be attributed to an increase in translational entropy caused by the randomness of displaced water molecules from the surface of the adsorbent (Wright and Pratt, 1974). The negative values of entropy have been reported earlier in the
References
-
Ajmal, M., Rao, R. A. K., Ahmad, R., and Ahmad J. “Adsorption studies on Citrus reticulata (fruit peel of orange): removal and recovery of Ni (II) from electroplating wastewater”. Journal of Hazd. Materials, (2000) 49, 1-2, 117-131.
-
Ajmal, M., Rifaqat, A. K. R., and Bilquees, A. S. ​​​​​​​“Adsorption and removal of dissolved metals using pyrolusite as adsorbent”. Environmental Monitoring and Assessment, (1995) 38, 25-35.
-
Alaerts, G. J., Jitjatuarant, V., and Kelderman, P. ​​​​​​​“Use of coconut shell based activated carbon for chromium (VI) removal”. Wat. Sci. Tech., (1989) 21, 1701. (Quoted by Ramos, et al., 1994).
-
Arulnatham, A., Balasubramain, N., and Ramakrishna, T.V. Coconut shell for treatment of cadmium and lead containing wastewater. Metal finishing, (1989) 87, 51-55.
-
Ayub, S., Ali, S. I., and Khan, N. A. “ Efficiency Evaluation of Neem bark (Azadirachta indica) bark in the treatment of industrial wastewater “. Environmental Pollution Cont. Journal, (2001) 4(4), 34-38.
-
Ayub, S., Ali, S. I., and Khan, N. A. “Treatment of Wastewater by Agricultural Wastes”. Environmental Pollution Cont. Journal, (1998) 2 (1).
-
Ayub, S., Ali, S. I., and Khan, N. A. “Extraction of Chromium from the wastewater by Adsorption”. Environmental Pollution Cont. Journal. (1999) 2(5).
-
Ayub, S., Ali, S. I., and Khan, N. A. “Adsorption studies on the low cost adsorbent for the removal of Cr (VI) from the electroplating industries”. Environmental Pollution Cont. Journal, (2002) 5 (6).
-
Ayub, S., Ali, S. I., and Khan, N. A. “Chromium removal by adsorption on coconut shell”. Journal of Indian association for Environmental Management, NEERI, (2003) 30.
-
Baisakh, P. C., Patnaik, S. N. “ Removal of Hexavalent chromium from aqous solution by adsorption on coal char”. Ind. J. of Env. Hlth, (2002) 189-196.
-
Baisakh, P. C., Patnaik, S. N., and Patnaik, L. N., “Removal of COD from textile mill effluent using flyash”. Ind. J. Env. Protection, (1996) 16 (2), 135-139.
-
Bansal, T. K., and Sharma, H. R. “Chromium removal by adsorption on rice husk ash”. Ind. J. Environ. Protec., (1992) 12,198 (Quoted by Rai, et al., 1995).
-
Browing, E. “Toxicity of industrial metals”. Bullerworths and Co., London (Quoted by Huang and Wu, 1975) (1969).
-
Chand, S., Aggarwal, V. K., Kumar, P. “Removal of Hexavelent Chromium from the Wastewater by Adsorption”. Indian J. Environ. Hlth. (1994) 36, 3, 151-158.






