Phytochemical Estimation of the Biocrude of Pedilanthus tithymaloides- A Petrocrop with Pharmacognostic Properties
1
Department of Botany,
M.C.C. Government College,
Abu road,
Rajasthan
India
Corresponding author Email: dranshursaxena@gmail.com
DOI: http://dx.doi.org/10.12944/CWE.19.1.32
Copy the following to cite this article:
Rani A. Phytochemical Estimation of the Biocrude of Pedilanthus tithymaloides- A Petrocrop with Pharmacognostic Properties. Curr World Environ 2024;19(1). DOI:http://dx.doi.org/10.12944/CWE.19.1.32
Copy the following to cite this URL:
Rani A. Phytochemical Estimation of the Biocrude of Pedilanthus tithymaloides- A Petrocrop with Pharmacognostic Properties. Curr World Environ 2024;19(1).
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2023-10-18 |
|---|---|
| Accepted: | 2024-04-22 |
| Reviewed by: | 
 Tridip Boruah
Tridip Boruah
|
| Second Review by: |

 N. Praveen Kumar
N. Praveen Kumar
|
| Final Approval by: | Dr Gopal Krishan |
Introduction
Increasing energy demands due to rapid industrialization, expansion of agriculture and enormous increase in population have not only resulted in the extensive depletion of non-renewable petroleum resources but also increased global warming and climate change. This environmental deterioration and loss of fossil fuels has promoted the research in search of alternative renewable energy. Hence, it is essential to look for new sources of organic compounds which can act as substitute of petroleum-based substances.1,2 These sources are plants which are called Petrocrops as they produce petroleum substances as supplementary to petrol. These plants produce hydrocarbon compounds or ethanol. Calotropis procera, Pedilanthus tithymaloides, Copifera langsdorfii, Euphorbia lathyris and Jaropha curcas are examples of petro-crops. It has been reported that gasoline and identical compounds are obtained from plant hydrocarbons with the help of catalyst like Zeolite (ZSM-5 type).3,4In Grindelia squarrosa (Pursh) whole plant methylene chloride extract has been reported to be highly converted into liquid fuels4. Such closely developed methods change the scope of fuels and biochemicals.
Most of the phytochemical crop studies by Calvin,5 are based on latex- bearing species, particularly Euphorbia species that can also be grown in arid and semi-arid regions of the world. A popularly known ornamental plant Pedilanthus tithymaloides (L.) Poit. (Euphorbiaceae), new scientific name Euphorbia tithymaloides (L.) Poit. is a succulent shrub commonly found in America and Asia. It is 0.4m to 3m tall and 40-60 cm wide. Some common names for Pedilanthus tithymaloides include Red Bird Flower, Devil's Backbone, Bock thorn, Christmas Candle, Fiddle Flower, Slipper Plant, Violin Flower, and so on. It thrives in various soil types, especially those with abundant micronutrients like Boron, Copper, Iron, Manganese, Molybdenum and Zinc. while it struggles to grow in highly saline soil. The plant can flourish when supplemented with fertilizers. Stem is olive-green with ovate leaves and beak-shaped flowers. laticifers are present in the whole plant. It is known as petro-crop and has medicinal importance also. Investigations have been carried out in the field of developing agro-technology for enhancing biomass and bio crude productivity of Pedilanthus tithymaloides.6,7,8,9,10 There was a need to develop proper extraction technique and to identify the hydrocarbon compounds present in the plant. Research was carried out on extraction procedure of hydrocarbons in the University of Arizona. Analysis of latex has revealed the presence of a large number of secondary metabolites in Calotropis procera 11,12,13,14,15,16 Euphorbia sikkinensis Boiss 17Croton bonplandianum Baill18 , Euphorbia hirta 19,20,21,22Euphorbia lathyris 23Major classes of hydrocarbon like substances present in bio-crude include terpenoid, isoprenoid, polymers and long chain aliphatics. Hydrocarbons from Pedilanthus macrocarpus have been analyzed by H’-NMR indicating the alkane range C27H56 to C35H7224. Gas-liquid chromatography of E. antisyphilitica revealed the presence of C29H60, C31H62 and C33 H68 compounds24. A new tetracyclic triterpene (3-epi-cyclolaudenal-22-en-3 alpha-ol) cyclolaudenol has been reported in E. cauducifolia.25Alcoholic extract of roots of E. ferganensis B. Fedtsh yielded on chromatography an aromatic ethyl ether (C29 H1008, m.p. 158-160 degree Celsius) and a coumarin, scopoline .26
Phytochemical analysis was carried out on P. tithymaloides var. green, var. cuculatus and var. variegatus to find out the occurrence of various metabolites that might have potential pharmaceutical applications as well as use in biomass derived hydrocarbon like material of high-grade transportation fuel.
Materials and Methods
Plants of P. tithymaloides var. green, var. cuculatus and var. variegatus were harvested in the month of August and identified in the herbarium of Rajasthan University. Aboveground portions were dried and powdered. Thimbles of this powder were used for soxhlet extraction using low-boiling nonpolar hexane as solvent. The hexane extract of P. tithymaloides var. green obtained was then fractionated using the column chromatographic technique. Some of the fractions thus obtained were subjected to gas-liquid chromatography at the University of Rajasthan.
Column chromatography
Silica gel H Glaxo grade was used for the packing of the column measuring 1 cm x 60 cm. The bio crude obtained by soxhlet extraction was filtered twice or thrice on Whatman filters paper No. 1. The filtrates obtained were mixed with silica gel and loaded on a packed silica gel column. This was covered by a thin cotton layer. Different solvents were passed through the column serially according to their increasing polarity. The solvents used in the present study were hexane (having polarity 0.0), benzene (having polarity 2.7), ethyl acetate (having polarity 4.4), acetone (having polarity 5.1), methanol (having polarity 6.6) and chloroform (having polarity 9.4) in a sequence in the combination of pure solvent, 3:1, 1:1 and 1:3. Twenty-one fractions were collected from the extract based on colour.
Gas Liquid Chromatography
The fractions obtained from column chromatography were studied using the GLC technique. The chromatography used was Hewlett-Packard 5890A gas-liquid chromatography. 0.5µl fraction was injected on the 10-meter long HPL (Methyl Silicon gum column) of 0.53 mm diameter with a film thickness of 2.65 µm. The chart speed was 0.50-1.0 cm. per minute. Nitrogen was used as carrier at a flow rate of 40 ml/minute. The flow of hydrogen was 35 ml/minute and of air was 360 ml/minute. The column was initially set at 50?C and it gradually increased to a final temperature of 300?C at a rate of 15?C per minute.
Qualitative phyto-chemical estimation of biocrude and plant dried samples–
To estimate the occurence of different hydrocarbons in the biocrude and plant-dried samples qualitative phyto-chemical assay was carried out using established methods proposed by Gibbs27 and Harbone.28
Results and Discussion
In the present investigation ground plant parts including leaves and stems of all three varieties of Pedilanthus tithymaloides collected in urban areas were processed to obtain the profile of the phytochemicals present. The assay resulted in the detection of Flavonoids, Triterpenoids, Sterols, Alkaloids, Anthraquinones and Saponins. However, Tannins, Iridoids, Juglone, Leucoanthocyanins and Catechol were not detected (Table 1).
Column chromatography fractions of the bio crude when analyzed through GLC revealed peaks at different retention times for a large number of compounds that need further characterization. Fraction obtained in hexane: benzene (1:1) showed four major peaks at retention time 4.90, 5.10, 6.76 and 8.86 minutes at chart speed 1.0 cm/per minute. Fraction obtained in benzene and fraction obtained in benzene: ethyl acetate in the ratio of 1:1 and 1:3 showed various peaks. Major peaks obtained were at 2.98, 4.36, 5.71, 6.79, 8.76 and 9.11 minutes at chart speed.1.0 cm/min. in the fraction obtained in pure ethyl acetate. Gas-liquid chromatograms (1 -5).
Table 1: Response of Pedilanthus tithymaoloides var. green, var. cuculatus and var. variegatus to different phytochemical tests.
Phytochemicals | var. green | var. cuculatus | var. variegatus |
Tannins | Not existing | Not existing | Not existing |
Anthraquinones | Existing | Existing | Existing |
Triterpenoids | Existing | Existing | Existing |
Sterols | Existing | Existing | Existing |
Alkaloids | Existing | Existing | Existing |
Flavonoids | Existing | Existing | Existing |
Iridoids | Not existing | Not existing | Not existing |
Juglone | Not existing | Not existing | Not existing |
Leucoanthocyanins | Not existing | Not existing | Not existing |
Saponins | Existing | Existing | Existing |
Catechol | Not existing | Not existing | Not existing |
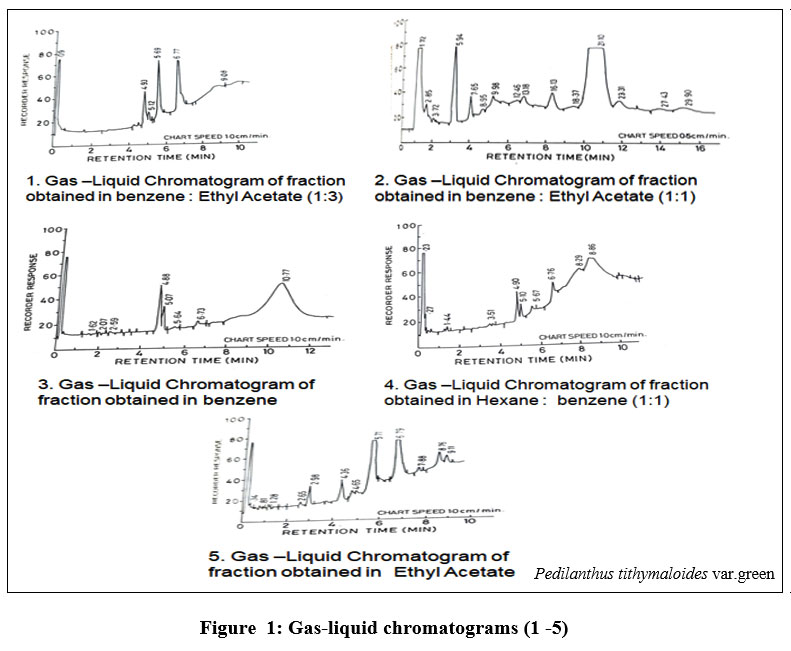 | Figure 1: Gas-liquid chromatograms (1 -5).
|
Other workers also found similar results as hexane, ethyl acetate and ethanol extracts from leaves of Pedilanthus tithymaloides were tested phytochemically resulting in the detection of triterpenes, steroids, saponins, tannins and coumarins which were effective for antimicrobial activities and other biological properties.29,30,31. Sandjo and coworkers32 reported that nine coumarin derivatives derived from Pedilanthus were able to stop the germination of Magnaporthe orizae a phytopathogenic sporulating fungus. Antimicrobial activities of diterpenoids extracted from Pedilanthus tithymaloides were reported to be effective against Mycobacteryum tuberculosis .33 The major components extracted from Euphorbia latex were tetracyclic triterpenoids mainly cycloartenol, 24-methylene cycloartenol and lanosterol. 34 It was reported that the latex of E. lathyris converts acetate and mevalonate to many tetracyclic triterpenes, mainly triterpenols and their fatty acid esters which are structurally different.35,36 Detailed investigations carried out to find out the source of triterpene biosynthesis have revealed that an osmotically sensitive organelle of the plant cell and possibly calmodulin are involved in the triterpene biosynthesis. 34,37,38,39
The experiments show that triterpenol and triterpene esters are synthesized at separate sites in a plant. Triterpene ester with triterpenols of the latex are synthesized in the wall lining cytoplasm of the laticifers in Hoya diversifolia Blume. and other Hoya species, (Asclepiadaceae), 40,41,42
An assortment of solvents were utilized to extract pertinent phytochemicals, such as coumarins, tannins, steroids, triterpenes, and saponins from hexane and ethyl acetate extracts43. Kaempferol 3-O-o-D-glucopyranoside-600-(3-hydroxy-3-methylglutarate), quercitrin, isoquercitrin, and scopoletin were the antioxidants found in a bioassay. A total of 76 point 0 mg of gallic acid equivalents/g extract 44 was determined to be the phenolic and flavonoid contents. Pedilanthus tithymaloides was found to contain a set of four coumarin derivatives.45 Steroids, cardenolides, anthraquinones, alkaloids, unsaturated steroids, phenolics, leucoanthocyanin etc. were reported from Pedilanthus tithymaloides.46 Plant chemicals such as flavonoids, terpenoids, phenols, tannins, saponins, glycosides, sterols, amino acids, and reducing sugars were found in the water filtrate of Pedilanthus leaves and stems. These secondary metabolites are useful in the creation of substitute bio-control agents that target mosquito vectors and agricultural insect pests.47
Twenty-one latex-bearing plants in the Indian state of Maharashtra were the subject of analysis. It was found that the secondary metabolites of these plants, which included Pedilanthus tithymaloides, were primarily alkaloids, flavonoids, terpenoids, cynogenic glycosides, phenolics, tannins, and saponins. These findings are consistent with the current study. The main finding was that all latex-bearing plants possessed pharmaceutically important secondary metabolites.48 Hence, the experimental plant has pharmacognostic and energy potential.
Conclusion
The findings derived from phytochemical analysis of the three varieties of P. tithymaloides revealed that the plants possess flavonoids, anthraquinones, sterols, alkaloids, triterpenoids and saponins. In order to determine the presence of gutta, rubber, waxes, and other materials, a gas-liquid chromatogram of hydrocarbon compounds extracted in hexane, benzene, and ethyl acetate revealed a number of peaks that could be further identified by 1H NMR analysis, 13 C-NMR analysis, IR spectroscopy, Proton Magnetic Resonance (PMR) and Gel Permeation Chromatography (GPC). The plant species is a very powerful energy source because of the plant's readily extracted compounds, such as triterpenoids, which can be cracked to produce high-octane gasoline. It can be used as a feedstock for fuel, chemicals, and petroleum derivatives. All three varieties are promising for development as petrocrops as they provide high biomass and high hydrocarbon yield. Evidence from the literature also justifies the pharmacognostic potential of the plant for treating various ailments.
Acknowledgements
The author would like to acknowledge the support of Rajasthan University Jaipur and his work place.
Funding Sources
The author received no financial support for the research, authorship and/or publication of this paper.
Conflict of Interest
The author doesn’t have any conflict of interest in the research.
Author’s Contribution
The research and paper writing was performed by the author itself.
Data Availability Statement
The manuscript incorporate all datasets produced or examined throughout this research study.
Ethics Approval Statement-
Experiment on no human or animal was performed during the study.
References
- Baas W.J. Investigations of leaf waxes III. Pentacyclic triterpenes, seco-triterpenes and nonvolatile aliphatics of four Hoya species and Ficus benjamina in relation to leaf age. Act. Bot. Neerl.1982; 31: 449-476.
CrossRef - Calvin M. Petroleum plantations for fuel and materials. Bioscience1979; 29: 533-53.
CrossRef - Gibbs R.D. Chemotaxonomy of flowering plants.McGill Queens Univ. Press, London; 1977.
- Govardhan C.H., Reddy P.R. and Sundararamaiah T. 3-epi-cyclolaudenol and known triterpenes from Euphorbia caducifolia. Phytochemistry1984; 23: 411-413.
CrossRef - Groeneveld H.W.1976. Biosynthesis of latex terpenes in Euphorbia, evidence for a dual synthesis. Acta Bot. Neerl.1976; 25: 459-473.
CrossRef - Groeneveld H.W. and Koning D.J. Biosynthesis of phytosterols and latex triterenes in Hoya Australis R. Br. Ex Trill and Hoya carnosa R. Br. Act. Bot. Neerl.1976; 25: 227-250.
CrossRef - Groeneveld H.W. and Vanderburg B. Quantitative aspects of triterpene synthesis in laticifers of Hoya diversifolia Bl. Plant Sci. Lett.1984; 33: 81-91.
CrossRef - Gupta D.R.and Garg S.K. 1965. A chemical extraction of Euphorbia hirta Linn. Bull.Chem. Soc. Japan.1965; 39: 2532-2534.
CrossRef - Haag W.O., Rodewald P.G. and Weisz P.B. Catalytic production of aromatics and olefins from plant material. In: Proc. 2nd Chem. Congr.Am. Chem. Soc. Las Vegas, Nev.1980 pp.63-76.
- Harborne J.B. Phytochemical methods. (Eds) G.W. Chapman and D.O. Hall. Pergamon Press, New York,1984.
CrossRef - Kamalakannan,S.,Madhiyazagan,P.,Dhandapani,A.,Murugan,K. and Barnard, D. Pedilanthus tithymaloides (Euphorbiaceae) leaf extract phytochemicals: toxicity to the filariasis vector Culex quinquesfasciatus (Diptera; Culicidae). Vector- Borne and Zoonotic Diseases. 2010;8 :817-820.
CrossRef - Khan A. Q. and Malik A. A steroid from Calotropis procera. Phytochemistry. 1989;28: 2859-2861.
CrossRef - Matisui E.da S., Perrone, L.A., Araujo,F.A.M., Santos, A.L.M.D. and Lucena, J.M.V.M. Pedilanthus tithymaloides (L.) Poit: phytochemical prospection and antimicrobial activity.Scientia Amazonia, 2017; 6 (3): 53-57.
- Mc Laughlin S.P. and Hoffmann J.J. Survey of biocrude producing plants from South West. Econ. Bot. 1982;36: 323-339.
CrossRef - Mogkolvisut W. and Sutthivaiyakit S. Antimalarial and antituberculosis poly-o-acylated jatrophane diterpenoids from Pedilanthustithymaloides. Journal of Natural Products, 2007;70: 1434-1438.
CrossRef - Nemethy E.K., Otvos J.W. and Calvin M. Analysis of extractables from one Euphorbia. J. Am. Oil. Chem. Soc. 1979;56: 957-960.
CrossRef - Nemethy E.K., Skrukrud C., Piazza G.J. and Calvin M. Terpenoid biosynthesis in Euphorbia latex. Biochem. Biophys. Acta.1983;760: 343-349.
CrossRef - Pant R. and Chaturvedi K. a. Occurrence of lupeol in Calotropis procera latex. Curr. Sci.1989; 38: 302-303.
- Pant R. and Chaturvedi K. b. Chemical analysis of C. procera latex. Curr. Sci. 1989;38: 740-742.
- Pant R. and Chaturvedi K. c. Isolation of a new triterpene from Calotropis procera latex. Curr. Sci. 1989;38:1093-1094.
- Piazza G. J., Saggese E. J. and Spletzer K.M. a. Triterpene biosynthesis in the latex of Euphorbia lathyris, effect of calmodulin antagonists and chlorinated phenoxy compounds. Pl. Physiol. 1987;83: 177-180.
CrossRef - Piazza G. J., Saggese E. J. and Spletzer K.M. b. Triterpene biosynthesis in the latex of Euphorbia lathyris, calmodulin antagonists are infective in whole latex. Pl. Physiol. 1987;83: 181-184.
CrossRef - Ponsinet G. and Ourisson G. Aspects particuliersde la biosynthese des triterpenes dans le latex d’ Euphorbia. Phytochemistry 1968;7:757-764.
CrossRef - Pradhan B.P. and Khastgir H.N. Terpenoids and related compounds: Part IX. Chemical investigation of Euphorbia siklhimensis Bioss. J. Indian Phytopath. 1969;40: 491-494.
- Prakash N.K.U., Ranjithkumar M., Sripriya N., Lakshmi R.P., Deepa S. and Bhuvaneswari S. Antioxidant, free radical scavenging activity and CG-MS studies on Pedilanthus tithymaloides (L.) Poit. International Journal of Pharmacy and Pharmaceutical Science, N. 2014;6: 284-287.
- Rani A and Chouhan H.S. influence of growth regulators on biomass and hydrocarbon yield of Pedilanthus tithymaloides. International Journal of Green and Herbal Chemistry. Sec.A. Vol.5, N 2016; 2: 168-171.
- Rani A. and Kumar A. Comparative study on biomass production and hydrocarbon yield of three different varieties of Pedilanthus tithymaloides. Acta. Ecol. 1992;14: 77-79.
- Rani A. and Kumar A. Effect of edaphic factors on the growth and physiology of P. tithymaloides var. green. J. of Environment and Pollution 1995;2 (10) :5-8.
- Rani A., Roy S. and Kumar A. Effect of Salinity stress on growth and hydrocarbon yield of Pedilanthus tithymaloides. var. green. J. of Environment and pollution 1996;3 (1) :21-26.
- Sandjo L. P., Foster A. J., Rheinheimer J. Anke, H. Opatz, T. and Thines E. Cumarin derivatives from Pedilanthus tithymaloides as inhibitors of conidial germination in Magnaporthe orizae. Tetrahedron Letters 2012;53: 2153-2156.
CrossRef - Saxena A.R. Impact of farm yard manure on agronomy of Pedilanthus- a potential Petro crop. IJCRT 2018;6(1):676-678.
- Saxena V.K. and Saxena Y.P. Isolation and study of a triterpenoid from Calotropis proceraspp. J. Res. Ind. Med. Yoga and Homeo. 1979; 14: 2.
- Sternburg C. and Rodriguez E. Hydrocarbons from Pedilanthusmacrocarpus( Euphorbiaceae) of Baja California and Sonora, Mexico. Am. J. Bot. 1982;69: 214-218.
CrossRef - Tadshibaev M.M. and Lutfullin K.L. Phenol compounds of Euphorbia ferganensis Khim. Prir. Soedin. 1985;4: 571-572.
CrossRef - Tiwari K.P., Choudhary R.N. and Pandey G. D. 3- methoxy-4,6-dihydroxy-morphinandien-7-one. An alkaloid from Croton bonplandianum. Phytochemistry. 1981;29: 863-864.
CrossRef - Vasudevan P. and Giridhar. Calotropis procera (Ait.) R. Br.-a potential petro- crop. In: Proc. Workshop on Petro-crops, DNES, New Delhi. 1986;27-44.
- Wang S. and Huffman J.B.. Botanochemicals: supplements to petrochemicals. Econ. Bot. 1981;35: 369-382.
CrossRef - Warnaar F. Conjugated fatty acids from latex of Euphorbia lathyris. Phytochemistry. 1981;20: 89-91.
CrossRef - Weisz P.B., Hang W.O. and Rodewald P.G. Catalytic production of high – grade fuel (gasoline) from biomass compounds by shape- selective (catalysis). Science. 1979;206: 57-58.
CrossRef - Yoshida T., Chen L., Shingu T. and Okuda T. Tannins and related polyphenols of Euphorbiaceous plants IVth. Euphorbins A and B, novel dimeric dehydroellagitannin from E.hirta L. Chem. Pharm. Bull. 1988; 36: 2940-2949.
CrossRef - Yoshida T, Namba O, Chen L and Okuda T. a.Tannins and related polyphenols of Euphorbiaceous plants Vth. Euphorbins C and equilibrated dimer dehydroellagitannin having a new tetrameric galloyl group. Chem. Pharm. Bull. 1990;30: 86-93.
CrossRef - Yoshida T., Namba O., Chen L., and Okuda T. b. Euphorbin E, a hydrolysable tannin dimer of highly oxidized structure from E. hirta.Chem. Pharm. Bull. 1990; 38:1113-1115.
CrossRef - Khare. Indian Madicinal Plants: An illustrated Dictionary.2007,p.469.
CrossRef - Abreu P, Mathew S, Gonzalez T, Vanickova L, Costa D, Gomas A . Isolation and Identification of antioxidants from Pedilanthus tithymaloides. Journal of Natural Medicines.2008; 67:62-67.
CrossRef - Srivastava R and Soni N. Anupdated review on phytopharmacological profile of Euphorbia tithymaloides( L. ) Poit. The Pharma Innovation Journal 2019; 8(5): 109-115.
- Kothale KV, Rothe SP, Pawade PN, Phytochemical screening of some Euphorbiaceae members.Journal of Phytology. 2011;3(12) : 60-62.
- Kamalakannan S, Madhiyazhagan P, Dhandapani A, Murugan K, Barnard D. Pedilanthus tithymaloides(Euphorbiaceae) Leaf Extract Phytochemicals: toxicity to the Filariasis vector Culex quinquefasciatus (Diptera: Culicidae) Vector-Borne and Zoonotic Diseases, 2010; 10:8
CrossRef - Mahajan RT, Badgur SB. Phytochemical Investigations of some Laticiferous Plants belonging to Khandesh Region of Maharashtra. Ethnobotanical leaflets, 2008;12:1145-1152.






