Distribution of Recent Benthic Foraminifera from the Outer Channel in and Around Gabakund Sea Mouth of Chilika Lagoon
1
Department of Geology,
Utkal University, Vani Vihar,
Bhubaneswar,
Odisha
India
Corresponding author Email: kirtigeology@gmail.com
DOI: http://dx.doi.org/10.12944/CWE.19.1.29
Copy the following to cite this article:
Mallick K. R, Nayak P. K, Prusty S. Distribution of Recent Benthic Foraminifera from the Outer Channel in and Around Gabakund Sea Mouth of Chilika Lagoon. Curr World Environ 2024;19(1). DOI:http://dx.doi.org/10.12944/CWE.19.1.29
Copy the following to cite this URL:
Mallick K. R, Nayak P. K, Prusty S. Distribution of Recent Benthic Foraminifera from the Outer Channel in and Around Gabakund Sea Mouth of Chilika Lagoon. Curr World Environ 2024;19(1).
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2023-11-12 |
|---|---|
| Accepted: | 2024-04-10 |
| Reviewed by: | 
 Sakineh lotfinasabasl
Sakineh lotfinasabasl
|
| Second Review by: |

 Michael A. Kaminski
Michael A. Kaminski
|
| Final Approval by: | Dr. Gopal Krishan |
Introduction
Benthic Foraminifera belonging to the kingdom Protista thrive in specific ecological niches. These tiny microscopic organisms are characterized by extensive and continuous fossil records from Cambrian to recent times is used to study various past and present environmental conditions mostly of coastal ecosystems such as estuaries, lagoons, mangroves, backwaters etc. 1, 2. The calcareous and agglutinated test of benthic foraminifera incorporates changes in biological and physicochemical characteristics such as seawater temperature, pH, salinity, dissolved oxygen (DO), food availability etc. of the surrounding environment as they have high preservation potential3. They are susceptible to minimal changes in environments4 and store the information in their tests for example; they develop deformed tests in a stressful environment. Therefore the study of features of benthic foraminiferal tests, morphology, abundance, isotope composition throws light upon paleoclimate and paleoceanographic changes like sea level, monsoon variability, salinity, temperature, etc. Similarly, the application of these microfauna assemblage to address environmental parameters of the coastal ecosystem is immense2.
The physico-chemical parameters (pH, salinity, dissolved oxygen content, turbidity etc.) are affected by sediment and fresh water mixing, by change of seasonality because of the influence of continent and marine realm due to dynamic nature of coastal water bodies5. Because of the continuity of the aquatic habitat, the interaction between sea and water bodies from nearby landmasses draining into the lagoon is dynamic and makes the coastal lagoon mostly a complex environment. Factors like distance of the sea from the lagoon, tidal currents strength, wind driven currents, hydrodynamic turnover time, sediment nature, organic matter, loss of water by evaporation and gravitational circulation controls major changes in chemical and physical parameters of the lagoon6.
In the maritime state Odisha, a long and vast coastline of 485 kilometer is bestowed with unique and natural estuaries, delta, mangrove systems, and lagoons. Chilika (19°28’ to 19°54’ N; 85°54’N to 85°38’ E) covering the borders of Khordha, Ganjam and Puri district is not only the biggest coastal water body situated on India’s Eastern Coast but it’s also Asia’s biggest tropical coastal lagoon7. In recent times the water spread area is estimated to be at 704 km2 and 1,020 km2 whereas past estimates reported 905 km2 and 1,165 km2 during the summer & monsoon time respectively8, 9.
This study aims to assess the benthic foraminiferal relative abundance and the response of physico-chemical parameters on their population during pre-monsoon time at the new mouth opening in and around Gabakund.
Study Area
The lagoon includes a combination of fresh river water and marine brackish waters. This pear shaped wetland having dimensions sixty five kilometer and eighteen kilometer in length and width respectively is connected to Bay of Bengal by a long tapered strip of land through which a channel opens to the lagoon’s central sector10.
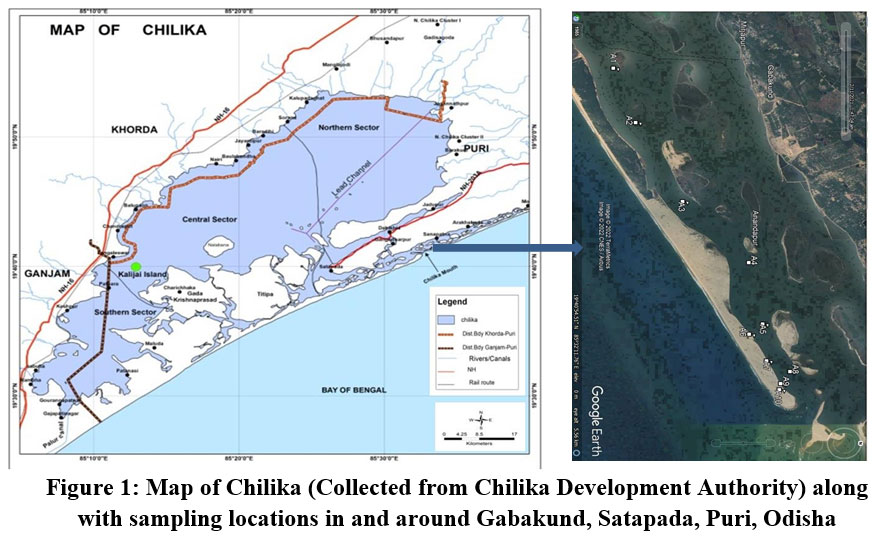 | Figure 1: Map of Chilika (Collected from Chilika Development Authority) along with sampling locations in and around Gabakund, Satapada, Puri, Odisha.
|
The lagoon is continuously influenced by three major sources of water systems; the Mahanadi river system, the Bay of Bengal and rivers (from western side of lagoon). The river systems (including western side rivers) include around 52 channels which serve as the source of fresh water to the lagoon. The basin of Chilika spreads over an area of 4300 km2 including the lagoon11. On basis of multiple geological and geomorphic parameters divided the lagoon into 4 sectors, i.e., the Northern sector, the Southern sector, the Central sector & narrow Outer channel12. The saline water incursion along the inlet region (sea mouth) to the lagoon have developed a niche for benthic foraminifers found in northern and central sectors under the influence of wind induced water inflow from Bay of Bengal. The sampling stations have been ideally chosen along the outer channel with considerable distance to overcome the physico-chemical parameters of the Lagoon.
During summer (April to June), elevated evaporation shrinks the thin water body whereas in time of monsoon which extends from July to September & post monsoon which is from October to November, lagoon swells up by large inflow of freshwater from different channels that drain into it13. The lagoon’s catchment area spread across 4406 square kilometers in which catchment from western side contributes 68% while the Mahanadi delta system contributes 32% and the total inflow of freshwater approximated around 14, 331 million km3/year14. Daya, Bhargavi, and Nuna are the three main distributaries of river Mahanadi contributes around 55% of the total discharge and small rivers from the western catchment accounts for 45%15. With time, due to heavy siltation and decrease in salinity and increased weed spread made the health of the brackish water lagoon in unsuitable conditions.
Climate
The mountain ranges of Eastern Ghats, extreme weather events occurring in Bay of Bengal over the time and regular tropical South West monsoon controls the ecology, environment and climate of Chilika lagoon. It is a hot, humid climate area with maximum temperature varying between 29?C–45?C whereas average rainfall of 1500 mm annually which is maximum from the month of June to September. The 100 km coastal shoreline experience around 10% of storm events originating from the Bay of Bengal which makes the 137 km barrier spit unstable and causes the mouth openings to move north. 16
Materials And Methods
Sediment samples of were taken from ten locations from the outer channel of lagoon Chilika in and around the Gabakund area (Fig.1). The sampling was done in the month of February, 2022. Using accepted international standard operating procedures for benthic foraminiferal studies, the samples were picked from different suitable locations where the chances of thriving of the species were more according to their niche of substrate. The exact locations of sampling were marked using a GARMIN Global Positioning System (GPS) instrument. The water parameters were recorded using a Hanna Multiparameter water quality sonde at each of the sampling locations.
Using a hand-held corer, the upper 2 cm of the sediment strata from one spatula (200gm) was initially taken out. The top (0–2) cm scoops of sediment from each core, constituting required sediment amount for study, were collected and processed using accepted international standard operating procedures17,18. 19.
Microscopic Analysis
Microscopic analysis was done with the stereo zoom binocular microscope (Nikon SMZ 745T) at the micropaleontology laboratory of Department of Geology, Utkal University. A significant amount of sand was studied under microscope to identify the calcareous microfauna. Identical species were kept in micropaleontological slides that were identified and counted at the species level. The analytical value of sand and clay contained were obtained and also marked the dominant sediment present in the sample. The characteristics of the shells were helpful in order to identify the environment/substrate from which these samples were collected.
Sediment fractions ? 500 ?m were examined for benthic foraminifera with large tests. The ?63 ?m sediments were studied for meiobenthic fauna. The Loeblich & Tappan (1988) monograph was used for identification of foraminiferal taxa upto the genus level19. Wet-picking representative of 63 ?m and ?150 ?m sieved fractions were used for species-level identification and Scanning Electron Microscope (SEM) imaging done. Such specimens obtained from wet picking were placed on an aluminum stub, and then the stub with the species was coated with gold-palladium for SEM imaging.
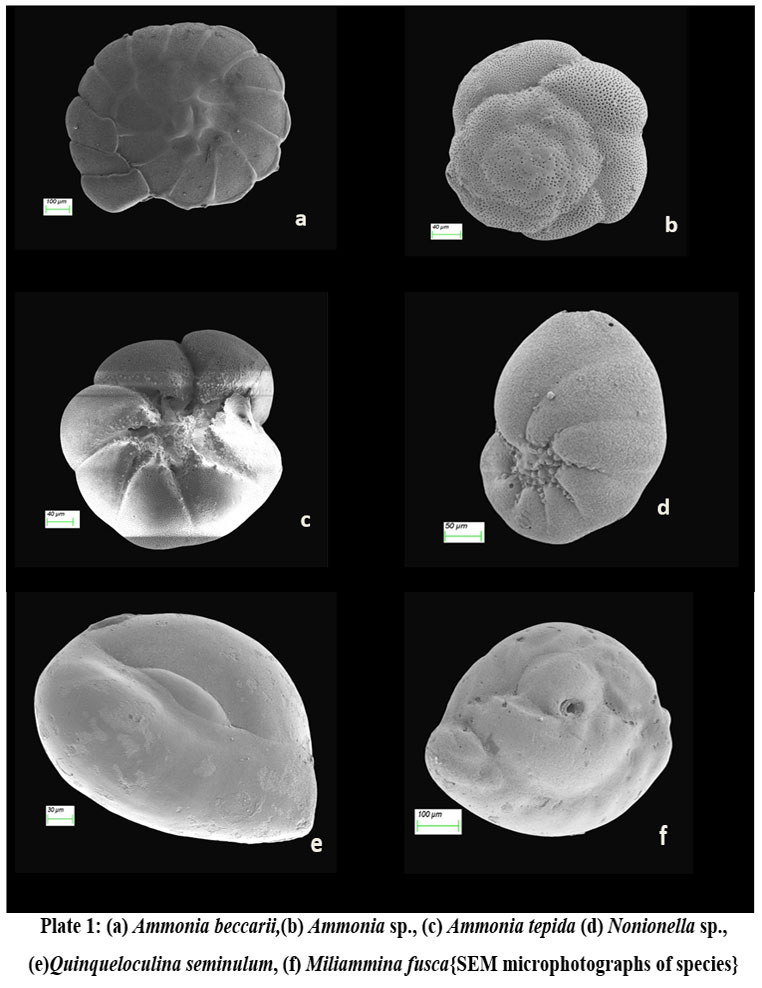 | Plate 1: (a) Ammonia beccarii,(b) Ammonia sp., (c) Ammonia tepida (d) Nonionella sp., (e)Quinqueloculina seminulum, (f) Miliammina fusca{SEM microphotographs of species}
|
Results And Discussion
The depth of Chilika lagoon varies from 0.3 m (dry season) to 1.8 m - 4.2 m (rainy season). Based on the physical and chemical parameters, the lagoon is subdivided into 4 sectors i.e. Southern sector, Northern Sector, Central sector, and the Outer Channel12. The lagoon gets connected with Bay of Bengal through Outer channel near Arakudha (old mouth) and at Gabakund (newly formed mouth). Brackish sea water comes into the Outer Channel through the old and new mouths and thus forms a typical estuarine to lagoon environment. The textural analyses of the sediments obtained at sampling stations (A1 to A10) were done applying Krumbein and Pettijohn20 method. Sand dominates as the substrate towards the mouth at Gabakund area and silty to clayey sand towards the southern part of the Outer Channel.
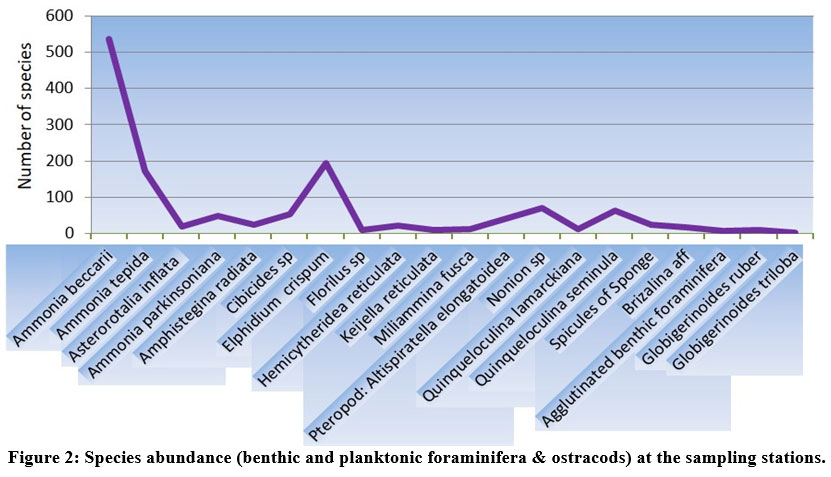 | Figure 2: Species abundance (benthic and planktonic foraminifera & ostracods) at the sampling stations.
|
Total of 36 species of benthic foraminifera belonging to 13 genera were documented at the sampling stations (Fig. 2). However, the species having highest abundance and also present in more than 4 sampling stations have been documented with the help of stereozoom microscope followed by SEM images.
The species abundance plot (Fig. 2) presents the average species composition at all 10 sampling stations. The abundant benthic foraminiferal species at all the sampled stations are Ammonia beccarii (Plate 1.a) and Ammonia tepida (Plate 1.c) (Table 1).These two species clearly show that salinity (25.53–27.86 psu) and temperature variability (24.96°C–27.16°C) across the sampling station doesn’t restrict their population (Table. 2). It can be interpreted that these two species are holeuryhaline species and are well adapted to salinity variations increasing with distance from the shore21. The ubiquitous A. tepida assemblage throughout the sampling stations is most prominent where phytoplankton provides copious labile carbon sources. The higher abundance of Ammonia sp. in the inner part of sampling stations corroborate a weak ocean current impact while the abundance of river water laden with nutrients ensures the sustainability of such genera.
Miliammina fusca (Brady 1870) (Plate 1.f) is a miliolid whereas its test is agglutinated. It got worldwide distribution mostly in habitats like dysoxic and mesohaline conditions: low salt marshes, swampy mangroves and estuaries of brackish origin 22, 23. The species unusual occurrence at adjacent locations (A5, A6, A7) in the outer channel of the lagoon is difficult to interpret. Perhaps the most possible explanation is the abundance of sandy substrate as the depth of the locations is shallow with low oxygen content. However the high influx of terrigenous input would favor the agglutinated taxa like M. fusca. In the sampling stations, the species richness is very low having a highest occurrence depicts a stressful environment for the occurrence of species to form its niche. Nonionella sp. (Plate 1.d) is found at a station in close proximity to the Gabakund mouth. The species have broad, low chambers which increase speedily whereas coiling is planispiral but involute. The species have rounded periphery, blazing test, umbilical region weakly depressed which loaded with granulated skeletal material, long sutures, calcareous made wall, finely perforate, granulated in structure, narrow interior marginal aperture have opening in equatorial region. Such species in particular indicate the depletion of calcium ion in the water and its preference to bind skeletal materials to form its test.
Quinqueloculina seminulum (Linnaeus, 1758) (Plate 1.e) is a cosmopolitan benthic foraminifer used in biomonitoring assessment and paleoenvironmental reconstructions24. The population of this porcelaneous species increases in the hyposaline condition which is evident in the present study as richness increased towards the sampling locations near the mouth opening which is a zone of intermixing of seawater with the lagoon.
Table 1: Number of various foraminiferal and Ostracod species found at the sampling locations of Chilika Lagoon (Outer Channel).
Sample location | A-1 | A-2 | A-3 | A-4 | A-5 | A-6 | A-7 | A-8 | A-9 | A-10 |
Species Name | ||||||||||
Ammonia beccarii | 106 | 57 | 22 | 76 | 48 | 56 | 36 | 49 | 48 | 38 |
Ammonia tepida | 17 | 14 | 22 | 11 | 29 | 12 | 22 | 18 | 18 | 09 |
Asterorotalia inflata | 0 | 0 | 1 | 2 | 1 | 2 | 2 | 5 | 2 | 4 |
Ammonia parkinsoniana | 12 | 0 | 9 | 0 | 6 | 9 | 9 | 4 | 0 | 0 |
Amphistegina radiata | 0 | 0 | 0 | 0 | 12 | 0 | 5 | 0 | 0 | 8 |
Cibicides sp | 0 | 12 | 0 | 0 | 12 | 6 | 7 | 4 | 6 | 7 |
Elphidium crispum | 28 | 22 | 19 | 21 | 9 | 12 | 23 | 32 | 6 | 22 |
Florilus sp | 0 | 0 | 0 | 4 | 0 | 1 | 1 | 4 | 0 | 0 |
Hemicytheridea reticulata | 1 | 0 | 1 | 0 | 3 | 1 | 0 | 6 | 4 | 5 |
Keijella reticulata | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 3 | 3 | 0 |
Miliammina fusca | 0 | 1 | 2 | 0 | 2 | 4 | 2 | 1 | 0 | 0 |
Pteropod:Altispiratella elongatoidea | 0 | 0 | 0 | 8 | 3 | 4 | 12 | 4 | 6 | 5 |
Nonion sp | 0 | 6 | 6 | 6 | 4 | 10 | 5 | 12 | 8 | 12 |
Quinqueloculina lamarckiana | 0 | 0 | 0 | 2 | 1 | 0 | 2 | 0 | 8 | 0 |
Quinqueloculina seminula | 2 | 0 | 0 | 4 | 1 | 8 | 4 | 9 | 22 | 12 |
Spicules of Sponge | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8 | 15 |
Brizalina aff | 0 | 0 | 0 | 7 | 2 | 1 | 4 | 0 | 0 | 2 |
Agglutinated benthic foraminifera | 0 | 0 | 0 | 3 | 1 | 3 | 0 | 0 | 0 | 0 |
Globigerinoides ruber | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 4 | 3 |
Globigerinoides triloba | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 |
Table 2: Depth, Salinity, Dissolved Oxygen (ppm) and Temperature records at the locations in Outer channel in and around Gabakund, Chilika Lagoon.
Sample location | Depth | Sal.[psu] | D.O.[ppm] | Temp.[°C] |
A1 | 1m | 25.53 | 6.98 | 24.97 |
A2 | 1.7m | 27.81 | 7.16 | 25.32 |
A3 | 1m | 27.84 | 7.16 | 25.28 |
A4 | 0.9m | 27.82 | 7.17 | 26.14 |
A5 | 0.8m | 27.78 | 7.12 | 25.74 |
A6 | 0.55m | 27.82 | 7.03 | 25.29 |
A7 | 0.55m | 27.73 | 6.30 | 25.56 |
A8 | 0.5m | 27.81 | 7.00 | 25.34 |
A9 | 0.6m | 27.79 | 6.38 | 25.63 |
A10 | 0.4m | 27.86 | 6.22 | 27.16 |
Site A5 to A8, most of the benthic foraminifera show a test color alteration. The environmental perturbation due to anthropogenic activities may be prime reason for such abnormalities in the test. In these three sampling station the average test size reduction is reported but the species diversity and richness is not affected.
Two species of planktic foraminifera, Globigerinoides ruber and Globigerinoides triloba have been encountered in the sediment samples at A8, A9 and A10. Their presence in the lagoon suggests their transport into the new inlet is through inflow of sea water due to prevailing winds from the Bay of Bengal by tidal currents during pre-monsoon season.
The Ostracods encountered in the study area of two different genera are moderately calcified, pitted or highly ornate forms. The species Keijella reticulate is found in the outer channel indicate a stress free turbulent beach like environment. During the taxonomic work the species of Ostracods of Hemicytheridea reticulate were found having isolated open valve in the benthic assemblages. After examination of theclosed carapace (70%) and open valve ratio (30%) (for two species of Ostracods) reveal rapid rate of sedimentation in the new mouth area near to the Bay of Bengal. However the species abundance is distributed throughout the sampling station but an increase in percentage of species towards the mouth region of the Gabakunda area is high.
The microphotograph of Pteropod Altispiratella elongatoidea is found in sample location (A4 - A10). In the sampling station A4 to A7 more than 50 percent ofthe same species is found with quartz grain being trapped in the operculum (Fig. 3). The attributes of each element in the EDS sensor obtained by the detector were studied. This is well established with the help of FESEM where the peak indicates the clogging with 31.6 percent of silica and 68.4 percent of oxygen as an individual entity.
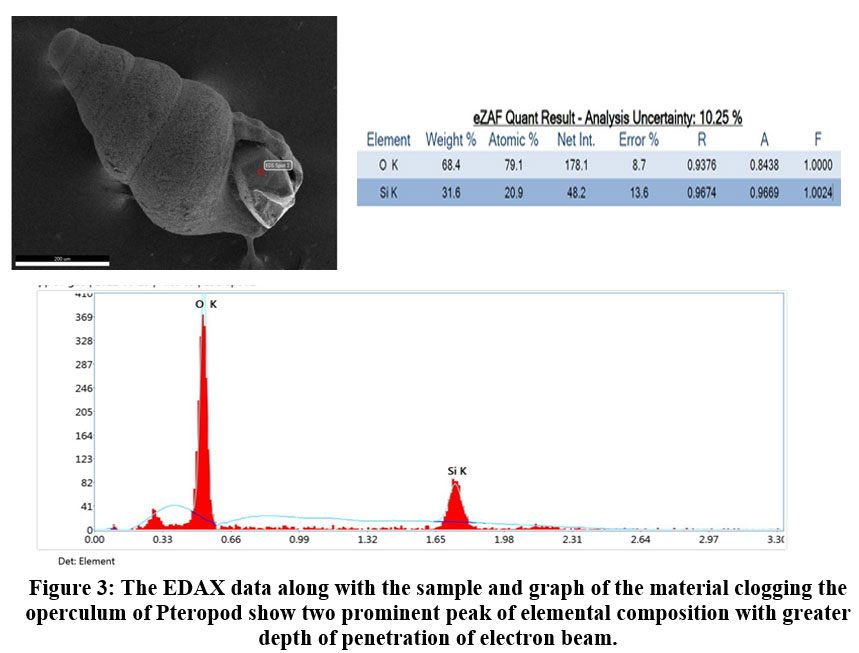 | Figure 3: The EDAX data along with the sample and graph of the material clogging the operculum of Pteropod show two prominent peak of elemental composition with greater depth of penetration of electron beam.
|
Conclusions
The present study throws light upon the benthic foraminiferal affinities, species richness and modalities for adaptability in Chilika lagoon which is characterized by natural and anthropogenic variabilities spatially. In the outer channel at Gabakund sea mouth calcareous benthic foraminiferal are dominant while few agglutinated foraminifera such as Miliammina fusca have found their unusual niche in the lagoon. Dominant species Ammonia beccarii and Ammonia tepida in all the sampling station with temperature and salinity variation indicate abundant labile carbon sources in the brackish water. In the zone of intermixing of sea and river water, population of cosmopolitan porcelaneous Quinqueloculina seminulum increase is significant. Abnormalities in test such as test size reduction and color alteration point towards environmental impact due to anthropogenic activities. Sparse presence of passive dwelling planktonic foraminiferal species in the study area can be attributed to prevailing wind induced currents from the Bay of Bengal towards the Chilika lagoon. The SEM microphotographs of the benthic foraminifera at Gabakund will support researchers to understand the taxonomic nomenclature of various species.
Acknowledgments
The authors would like to thank Department of Geology, Utkal University for all the laboratories facilities during the course of the present work.
Funding source
This work was supported by the OURIIP seed fund, Odisha State Higher Education Council (OSHEC), Government of Odisha (Grant No- 29seed/2019/Geology-1).
Conflict of Interest
No potential conflict of interest was reported by the author(s).
Authors’ Contribution
KRM conceived and designed the idea, KRM, PKN and SP collected the data, PKN and SP performed the analysis, KRM and PKN wrote the paper.
Data Availability Statement
The manuscript incorporates all datasets produced or examined throughout this research study.
References
- Saraswat, R., and Nigam, R. Benthic Foraminifera. Encyclopaedia of Quaternary Science ed. Elias, S.A. Amsterdam: Elsevier 2013.
- Yanko, V. Ahmad,M. Kaminski, M. Morphological deformities of benthic foraminiferal tests in response to pollution by heavy metals: implications for pollution monitoring J. Foraminifer. Res., 1999; 28; pp. 177-200
- Gupta, V. Sen, A. Pattnaik, A. Rastogi, G. Bhadury, P. Long-term monitoring of benthic foraminiferal assemblages from Asia's largest tropical coastal lagoon, Chilika, India. Journal of the Marine Biological Association of the United Kingdom. 2018: 99: 1-20. 10.1017/S0025315418000085.
CrossRef - Barik, S.S., Singh, R.K., Jena, P.S., Tripathy, S., Sharma, K., Prusty, P. Spatio-temporal variations in ecosystem and CO2 sequestration in coastal lagoon: a foraminiferal perspective. Mar. Micropaleontol. 2019:147: 43–56. https://doi.org/10.1016/j.marmicro.2019.02. 003.
CrossRef - Frontalini, F., Coccioni, R. Benthic foraminifera as bioindicators of pollution: a review of Italian research over the last three decades. Rev. Micropaleontol. 2011: 54: 115–127. https:// doi.org/10.1016/j.revmic.2011.03.001.
CrossRef - Chilika Newsletter, VII edition, 2013
- Gupta GVM, Sarma VVSS, Robin RS, Raman AV, Kumar MJ, Rakesh M, Subramanian BR. Influence of net ecosystem metabolism in transferring riverine organic carbon to atmospheric CO2 in a tropical coastal lagoon (Chilka Lake, India). Biogeochemistry 2008: 87:265 285
CrossRef - Annandale, N.& Kemp, S. Fauna of Chilka Lake. The Echiuroidea of the lake and of the Gangetic delta Memoirs of the Indian Museum. 1915: 5: 55-63
- Sahu B.K., Pati P. and Panigrahy R.C. Environmental condition off Chilika lake during pre and post hydrological intervention: an over view. Journal of Coastal Conservation.2014: 18, 285–297.
CrossRef - Das, N. K. and Samal, R. C. Environmental survey of Chilika. In: S. N. Patro (ed.) Chilika: the Pride of Our Wetland Heritage. Orissa Environmental Society, Bhubaneswar, India: 1988: 93–103.
- Kumar A, Equeenuddin SM, Mishra DR, Acharya BC. Remote monitoring of sediment dynamics in a coastal lagoon: longtermspatio-temporal variability of suspended sediment in Chilika. Estuar Coast Shelf Sci. 2016:170:155–172. https ://doi. org/10.1016/j.ecss.2016.01.018
CrossRef - Mohanty, P. K., S. K. Dash, P. K. Mishra and A.S.N. Murty. Heat and momentum flux over Chilika: A tropical Lagoon. Indian Journal of Marine Sciences. 1996: 25: 184-188.
- Panigrahi, S., J. Wikner, R. C. Panigrahy, K. K. Satapathy and B. C. Acharya. Variability of nutrients and phytoplankton biomass in a shallow brackish water ecosystem (Chilika Lagoon, India). Limnology.2009: 10(2): 73-85.
CrossRef - Anon. Protecting Lake Taupo. A Long Term Strategic Partnership. Environment Waikato, Hamilton. 2003
- Mishra, S. P. and Jena, J. G. Characteristics of western catchment and their inflow contribution to Chilika Lagoon, Odisha (India). International Journal of Lakes and Rivers. 2013: 6 (2): 119-129.
- Buzas M.A., Hayek L.C., Reed S.A. and Jett J.A. Foraminiferal densities over five years in the Indian River Lagoon, Florida: a model of pulsating patches. Journal of Foraminiferal Research. 2002: 32, 68–92.
CrossRef - Schönfeld J., Alve E., Geslin E., Jorissen F., Korsun S. and Spezzaferri S. The FOBIMO (Foraminiferal Bio-Monitoring) initiative – towards a standardised protocol for soft-bottom benthic foraminiferal monitoring studies. Marine Micropaleontology. 2012: 94, 1–13.
CrossRef - Loeblich, A.R. and Tappan, H. Foraminiferal Genera and Their Classification: Van Nostrand Reinhold Company, New York. 1988: 2 Volumes, 970 pp.
- Regulagadda, A. Biofacies of Abundant Foraminifera in Relation to Bottom Sediments in Outer Channel of Chilka Lake, Odisha, East Coast of India. Gondwana geological. 2013: V. 28(1), 69-75.
- Krumbein, W.C. and Pettijohn, F.J. Manual of Sedimentary Petrography. Appleton Century Crofts, New York. 1938: 549 p.
- Tyszka, J. "Miliamminagerochi n. sp.-a Middle Jurassic rzehakinid (Foraminiferida) from quasi-anaerobic biofacies." AnnalesSocietatisGeologorumPoloniae. 1997: Vol. 67. No. 2-3.
- Sen Gupta, B.K. Systematics of Modern Foraminifera. In: Sen Gupta, B.K., Ed., Modern Foraminifera, Kluwer Academic Publishers, Dordrecht. 1999: 7-36
CrossRef - Kaushik, Tushar, ThirumalaiMurugan, and Dagar, S.S. Morphological variation in the porcelaneous benthic foraminifer Quinqueloculinaseminula (Linnaeus, 1758): Genotypes or Morphotypes? A detailed morphotaxonomic, molecular and ecological investigation." Marine Micropaleontology. 2019:150 101748.
CrossRef






