Determination of Heavy Metals in Indoor Dust in the Vicinity of Kota Thermal Power Plant under Meteorological Influence at an Industrial City
1
Department of Chemistry,
Government College,
Kota,
Rajasthan
India
2
Department of Computer Science and Engineering,
Manipal University Jaipur,
Jaipur,
Rajasthan
India
Corresponding author Email: meenamanju87@gmail.com
DOI: http://dx.doi.org/10.12944/CWE.19.1.24
Copy the following to cite this article:
Meena B. S, Meena C, Hada P. S, Chandrawat U, Meena M. Determination of Heavy Metals in Indoor Dust in the Vicinity of Kota Thermal Power Plant under Meteorological Influence at an Industrial City. Curr World Environ 2024;19(1). DOI:http://dx.doi.org/10.12944/CWE.19.1.24
Copy the following to cite this URL:
Meena B. S, Meena C, Hada P. S, Chandrawat U, Meena M. Determination of Heavy Metals in Indoor Dust in the Vicinity of Kota Thermal Power Plant under Meteorological Influence at an Industrial City. Curr World Environ 2024;19(1).
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2024-02-01 |
|---|---|
| Accepted: | 2024-05-05 |
| Reviewed by: | 
 Abdulmutalib Raafat
Abdulmutalib Raafat
|
| Second Review by: |

 Mounia Tahri
Mounia Tahri
|
| Final Approval by: | Dr. P A Azeez |
Introduction
Indoor air pollution can result from the infiltration of outdoor pollutants (road dusts), comprising vehicular, household and factory releases, eroding of roadside construction and other sources. Dust comprises of particulate matter from organic/ inorganic constituents along with dry deposition, earth crust and other pollutants, particularly toxic heavy metals1-7. Incidental incorporation of dust facilitates entry of heavy metals in the body.
Road dust is made up of particulate matter (PM) comprising of fine residue (< 100 µm) driven through environmental or anthropogenic activities. It does not stay in one location for very long. Trace metals like Fe, Zn, Cu, Pb and Cd have toxicity at low concentrations are significantly contributed by resuspension of this road dust into the atmosphere8-11.
Overconsumption of non-essential trace elements, like copper, results in chronic poisoning, gastrointestinal issues, and liver damage. When iron (III) concentrations are higher, it is also hazardous 12. Lead is a heavy metal that impairs human blood, liver, kidney, and brain function. The majority of household and industrial paints include it13. Renal, endocrine, and reproductive disorders as well as malignancies can be brought on by cadmium (Cd), even at comparatively low concentrations14-16.
The meteorological parameters i.e. temperature differences, moisture content and relative wind speed are found to affect disposition of heavy metal attached with atmospheric particles and need to be observed constantly as they determine the dispersal of these metals from the major sources to the study area. Following buildup in the atmosphere, these heavy metals are introduced into the food stream affecting all flora and fuana17-20.
Large amounts of fly ash, which is a homogenous blend of different metallic oxides, is produced in Kota City by Kota Thermal Power Plant (KTPP). Numerous Kota stone manufacturers, among other small and large-scale enterprises, further escalate concentration of heavy metals.
Hence, we decided to carry out the current study with the primary goals: (i) To identify the crustal (Ca and Fe) and anthropogenic (Cu, Pb, Cd, and Zn) metal levels in indoor dust; (ii) To identify potential sources of heavy metals linked to indoor dust; (iii) To investigate how temperature, relative humidity, wind direction, and other meteorological factors affect heavy metal concentration levels besides distance from KTPP.
Materials and Methods
Study Area
Located on the Chambal River bank at eastern side, at 25°11 N and 75°51 E, Kota is a major industrial city of south Rajasthan and is the nation's power production centre owing to KTPP (Kota Thermal Power Plant). Its semi-arid climate lies between 7.2°C (in winter) and 46.6°C (in summer). This area produces a significant volume of slurry, mostly composed of oxides of Ca, Mg and Si, as a result of the excavation, cutting, and polishing of a well-known Kota stone from more than 200 stone units18.
Indoor dust collection and analysis
Through the Global Positioning System, 47 sampling sites were chosen as per some certified standards 21. Figure 1 shows location of all the sampling sites of Kota City chosen for the present study.
Sampling was performed in the summer months (March, April and May, 2022) and October, 2022 at selected 47 sites of Kota City. With the aid of household vacuum cleaners, 188 (47 samples * 4 months of sampling period) residential indoor dust samples (one sample from each sampling sites per month making it 47 samples per month) were collected from private homes' floors, lamp covers, uncleaned windowsills, and cabinet roofs. Each sample was placed in an unused dust container and the brush was cleaned beforehand using a plastic spatula. Each dust sample was collected and immediately placed into a brand-new, labelled airtight polyethene package for secure storage and transit 22.
 | Figure 1: Location of sampling sites of Kota city at the study area. |
Total heavy metal digestion
After the samples were run through 300 BSS (< 53 µm) sieves, they were digested for further process. Since the concentration of the metals in these samples determines their mode of occurrence, a digestion procedure was used to extract only the nitric acid soluble fraction 17.
Total metal extraction was carried out through Nitric acid (HNO3) digestion23. After digestion, the concentrations of chosen metals (Fe, Zn, Cu, Cd, and Pb) were measured using the Direct Air – Acetylene Flame method (AAS-Shimadzu-6300), with the Ca metal being measured using the Flame Photometer (Systronics – 128) method. With the use of quality control blanks, certified reference material, and internal standards, precision and accuracy of the analysis were kept under observation.
Monitoring of meteorological parameters
During the measurement period (March, 2022 to October, 2022), the Automated Weather Station (model number: DCPAWS02) installed at the Kota Aerodrome, India, provided the meteorological data as given in Table 1 and Figure 2. The data were recorded hourly and averaged across 24-hour of operation time of samplers.
Table 1: Meteorological conditions of Kota City during the sample collection
Meteorological conditions | Summer months |
Temperature (°C) | 31.64 ± 12.85 |
Relative humidity (RH) (%) | 38.31 ± 15.26 |
Wind speed (m/h) | 1.7± 1.2 |
Rainfall (mm) | 1.61 |
 | Figure 2: Wind rose in Kota city during the sampling period.
|
Statistical analysis
Statistics of metal concentration in indoor dust was carried out using MS-Excel 2021. Pearson corelation coefficient, Enrichment factor analysis and Principal component analysis using varimax rotation were performed from the data using SPSS 22.0.
Pearson corelation matrix analysis is employed to investigate the interrelationships among heavy metals in indoor dust and predict the possibility of a common source between them any significant positive correlation indicates their common source.
The Enrichment Factor Analysis (EF) facilitates the identification of anthropogenic sources for a given element in addition to its primary natural source and for evaluation of the contamination degree of heavy metal pollution Considering the fact that concentration of Ca is not anthropogenically altered, it has been utilized as a reference element for EF evaluations. 24-26 Following EF calculation formula has been applied:
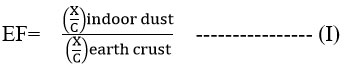
Here x and c are the concentration of the metal chosen and concentration of the reference metal respectively.
Principal component analysis is performed to identify the sources of heavy metal pollution. PCA aims at extracting the essential data as a set of variables which are termed as principal components, factor, eigenvectors or loading and finds the fewest major components explaining the statistical variance27-30.
Result and Discussion
Metal Concentrations
Indoor human actions and outdoor air characteristics (pollutant and aerosol infiltration) have a significant impact on indoor air quality. Additionally, fine particles from urban and industrial emissions, such as carbonaceous aerosols, have a higher metal load in comparison to earth dirt because of their tiny dimensions 31-33.
Present research paper focuses on determining the pollution load of copper, lead, cadmium, zinc, calcium, and iron under meteorological influence. The average concentration levels of Cu, Pb, Cd, Zn, Ca and Fe (Figure 3 to Figure 8) were highest in sampling sites S1(1.4796 mg/L), S1(3.1277 mg/L), S28(0.2316 mg/L), S1(5.8230 mg/L), S3(2306.72 mg/L) and S3(86.4095 mg/L) and lowest in S43(0.7353 mg/L), S43(1.2832 mg/L), S43(0.0874 mg/L), S43(3.5751 mg/L), S4(1591.58 mg/L) and S32(76.9529 mg/L) respectively.
The higher concentration of Cu, Pb, Zn at S1 and Cd at S28 sampling site is due to the closest distance of this site to the point source KTPP and lower concentrations of these metals are found at S43 sampling site due to its farthest location from the KTPP.
Moreover, the predominant direction of wind blow (North) as shown in Figure 2 was found to be mainly faced by S1 sampling site resulting in elevated concentration of anthropogenic origin metals at this site thereby making it high risk location from health point of view while these metals are found to be decreased at S43 due to location of this site falling in opposite direction of predominant wind blow (North).
During the sampling period, high average temperature (46.66°C), low relative humidity (38.31%) and high average wind speed (15km/h) (Table 1) caused lower concentrations of anthropogenic origin metals (Cu, Pb, Cd and Zn) while crustal origin metals (Ca and Fe) are found to be in higher concentration owing to their more deflation and transport caused by high wind strength34.
Ca and Fe, mostly found in the local soil, are mobilized or resuspended before being assimilated in the study area. The earth's crust and soil are alkaline sources of carbonates, which give rise to higher concentrations of Ca metal. On the other hand, Fe is found in oxide forms, such as goethite, hematite, and magnetite 35.
Cu, Cd, Zn, and Pb are caused by nearby industrial operations as well as coal combustion at KTPP. Being more volatile and elements with lower melting points, Zn and Pb are easily transported in the air by the power plant's coal combustion operations resulting in their enhanced concentrations36. Additionally, lubricants, rubber tire remnants, and corrosion of vehicle components could be the source of Zinc particles found in the ambient air 37. It is noteworthy here that lead has a longer half-life in the atmosphere than other elements besides being present in fly ash emissions from KTPP, its persistent presence in street dust is more likely the result of past vehicle emissions (before the ban of Pb-containing fuel)17. Accordingly, this study also shows Zn concentration as highest in the total fraction with lead, copper and cadmium in the subsequent order and are consistent with some earlier studies38-39.
 | Figure 3: Average concentrations of Cu metal in indoor dust gathered from various sampling sites during the sampling period.
|
 | Figure 4: Average concentrations of Pb metal in indoor dust gathered from various sampling sites during the sampling period.
|
 | Figure 5: Average concentrations of Cd metal in indoor dust gathered from various sampling sites during the sampling period.
|
 | Figure 6: Average concentrations of Zn metal in indoor dust gathered from various sampling sites during the sampling period.
|
 | Figure 7: Average concentrations of Ca metal in indoor dust gathered from various sampling sites during the sampling period.
|
 | Figure 8: Average concentrations of Fe metal in indoor dust gathered from various sampling sites during the sampling period.
|
Pearson’s Correlation Analysis
Table 2 shows Pearson’s correlation coefficients for selected heavy metals in indoor dust of Kota City.
Table 2: The results of Pearson’s correlation analysis for selected heavy metal concentrations in the indoor dust of Kota City (* significant at 5%)
Metal | Cu | Pb | Cd | Zn | Ca | Fe |
Cu | 1.000 |
|
|
|
|
|
Pb | 0.791* | 1.000 |
|
|
|
|
Cd | 0.812* | 0.839* | 1.000 |
|
|
|
Zn | 0.790* | 0.853* | 0.918* | 1.000 |
|
|
Ca | -0.471 | -0.615 | -0.592 | -0.687 | 1.000 |
|
Fe | -0.297 | -0.483 | -0.445 | -0.542 | 0.915* | 1.000 |
Table 2 shows a positive correlation between Pb – Cu (0.791), Cd-Cu (0.812), and Zn-Cu (0.790), Cd-Pb (0.839), Zn-Pb (0.853), and Zn-Cd (0.918) indicating a common source i.e. KTPP predominantly along with other industrial activities. Similarly, a positive correlation observed between Ca-Fe (0.915) indicates that both of these metals originate from natural soil.
Enrichment Factor
For the present study, EF values fall into these three categories: When EF values are less than 2 then it is insignificant enrichment; when it is in the range of 2-5 then enrichment is found to be moderate; with EF values lying in the range 5-20 then the enrichment is considered to be significant40-43.
Figure 9 to Figure 13 show the mean enrichment factors (EF) from the average heavy metal concentrations in the samples. The elements with the greatest EF values are found to be cadmium, copper, lead and zinc, all of which had EFs significantly more than 2. This implies a moderately anthropogenic pollution of the studied samples and the transportation of fly ash from coal combustion operations at KTPP can be associated with the significant EF of metals copper, cadmium, zinc, lead18.
 | Figure 9: Enrichment factor of Cu metal in indoor dust gathered from various sampling sites during the sampling period.
|
 | Figure 10: Enrichment factor of Pb metal in indoor dust gathered from various sampling sites during the sampling period.
|
 | Figure 11: Enrichment factor of Cd metal in indoor dust gathered from various sampling sites during the sampling period.
|
 | Figure 12: Enrichment factor of Zn metal in indoor dust gathered from various sampling sites during the sampling period.
|
 | Figure 13: Enrichment factor of Fe metal in indoor dust gathered from various sampling sites during the sampling period.
|
Principal component analysis
In the current work, PCA using varimax rotation was used to further identify the sources of heavy metals in indoor dust. Only two eigenvalues were >1, according to the results accounting for more than 91.30% of the variance. Two variables (varimax factors 1 and 2) account for all analyzed six metal species as shown in the rotated component matrix (Table 3).
With significant loading of heavy metals like Cu, Pb, Cd, and Zn, the first factor (VF 1), which accounted for approximately 55.84% of the variance, demonstrated the impact of anthropogenic activities, primarily coal burning at KTPP. High loading of Ca and Fe in VF 2, which explained 35.46% of the variance, implied the influence of crustal aerosols29,44.
Table 3: PCA displaying loading of 6 variables with two varimax factors (VF) in indoor samples.
Variables | Component | |
VF 1 | VF 2 | |
Cu | 0.922 | -0.112 |
Cd | 0.908 | -0.289 |
Pb | 0.867 | -0.335 |
Zn | 0.866 | -0.409 |
Fe | -0.184 | 0.967 |
Ca | -0.377 | 0.904 |
% of variance | 73.08% | 18.22% |
Cumulative (%) | 73.08% | 91.30% |
Conclusions
In this study, heavy metal pollution in indoor dust samples collected from 47 sampling sites in Kota City, Rajasthan during sampling period (March, April, May, 2022 and October, 2022) has been illustrated and threw a light on their possible sources and causes. During the sampling period, meteorological parameters such as speed and direction of wind, relative humidity, temperature were found to affect the concentration of metals. Decreased levels of anthropogenic metals are due to elevated average wind strength and average temperature and reduced relative humidity while owing to more deflation and transport of crustal origin metals caused by high wind strength resulted in their increased concentration.
Under abovementioned meteorological influence, on looking at the comparative order of concentration levels of selected heavy metal species at studied sites, it is observed that the closest distance of S1 sampling site to the point source KTPP along with the prevalent North direction of wind blow being faced by it has resulted in the elevated levels of anthropogenic origin metals Cu, Pb, Zn at this site making it high health risk location while Cd is found to be highest at S28 owing to its comparatively closer location to KTPP besides its having other anthropogenic features. On the other hand, these metals are found to be decreased at S43 due to its farthest distance from KTPP along with falling in opposite direction of predominant wind blow (North).
The heavy metals Cu, Cd, Zn and Pb are found to have common origins predominantly coal based KTPP along with moderate to significant enrichment by other vehicular and anthropogenic as indicated by EFs, positive correlations and Principal component analysis.
Hence, it is ascertained that coal burning at KTPP, prevalent direction of wind blow during the study period from the point source i.e. KTPP towards the studied sampling sites, their location with respect to the point source and other man-made characteristics of these sites are mainly responsible for the indoor heavy metal scourge in the studied area.
Human health is constantly threatened by this heavy metal pollution load in indoor household dust and further research regarding the exposure evaluation among the residents of an industrial town needs to be explored.
Therefore, assessment of heavy metal burden in Kota City is quite significant to avoid any potential health risks and appropriate decisions must be made by the administration considering the sources of heavy metal pollution while city planning.
Acknowledgements
The authors are thankful to CSIR for providing financial support for research work and DST FIST Laboratory Department of Chemistry, Government College, Kota for providing necessary facilities during the Research work.
Funding Sources
This work was supported by Council of Scientific & Industrial Research (CSIR Grant number/ File No: 08/0135(15103)/2022-EMR-I).
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions
The study is a part of research work being done by Chetan Meena, Research Scholar, Department of Chemistry, Government College, Kota under the joint research supervision of Professor Uttra Chandrawat (Co-supervisor) Department of Chemistry, Government College, Kota and myself as Research Supervisor while contributing in writing-original draft preparation, review and editing.
Dr. Bharat Singh Meena, Associate Professor, Department of Chemistry, Government College, Kota and Priyank Singh Hada, Faculty member of Department of Computer Science and Engineering, Manipal University Jaipur, Jaipur have provided their full support throughout the research work from sample collection, investigation and data analysis.
Data Availability
The research paper includes entire statistics information analysed during the research work.
Ethics Approval Statement
Not applicable.
References
- Ma X, Xia D, Zhang G, et al. Water-soluble ions and heavy metal levels, source apportionment, and health risk of indoor dust in the mogao grottoes of Dunhuang, China. Indoor Air. 2023;2023:1-21. doi:10.1155/2023/4818195.
CrossRef - Tariba Lovakovi? B, Jagi? K, Dvorš?ak M, Klin?i? D. Trace elements in indoor dust—children’s health risk considering overall daily exposure. Indoor Air. 2022;32(9):e13104. doi:10.1111/ina.13104.
CrossRef - Li X, Fan L, Wang X, et al. Characteristics, distribution, and children exposure assessment of 13 metals in household dust in China: A big data pilot study. Indoor Air. 2022;32(1):e12943, 1-18. doi:10.1111/ina.12943.
CrossRef - Shi T, Wang Y. Heavy metals in indoor dust: spatial distribution, influencing factors, and potential health risks. Sci Total Environ. 2021;755(1):142367. doi:10.1016/j.scitotenv.2020.142367.
CrossRef - Sabzevari E, Sobhanardakani S. Analysis of selected heavy metals in indoor dust collected from city of Khorramabad, Iran: A case study. Jundishapur J Health Sci. 2018;10(3):1-7.
CrossRef - Verma PC. Determination of concentration of some heavy metals in roadside dust in Damaturu metropolis which causes environmental pollution. Int J Adv Sci Eng Technol special issue. 2015;3:87-92.
- Turner A, Simmonds L. Elemental concentrations and metal bioaccessibility in UK household dust. Sci Total Environ. 2006;371(1-3):74-81. doi:10.1016/j.scitotenv.2006.08.011.
CrossRef - Nezhad MA, Ghasemi A, Mohtashami M, Saeidi J, Mashadrizeh AH. Heavy Metal Analysis in of Indoor and Outdoor Dust Extracts and cytotoxicity evaluation and inflammation factors on Lung, Gastric and Skin Cell Lines. Heliyon. 2022;8:1-7.
CrossRef - Dingle JH, Kohl L, Khan N, et al. Sources and composition of metals in indoor house dust in a mid-size Canadian city. Environ Pollut. 2021;289:117867. doi:10.1016/j.envpol.2021.117867.
CrossRef - Adekola FA, Dosumu OO. Heavy metal determination in household dusts from Ilorin City, Nigeria. Niseb J,. 2001;1(3):217-221.
- Ibanez Y, Le Bot B, Glorennec P. House-dust metal content and bioaccessibility: a review. Eur J Mineral. 2010;22(5):629-637. doi:10.1127/0935-1221/2010/0022-2010.
CrossRef - Vasu AE. Adsorption of Ni(II), Cu(II) and Fe(III) from aqueous solutions using activated carbon. J Chem. 2008;5(1):1-9. doi:10.1155/2008/690241.
CrossRef - Abia AA, Asuquo ED. Lead(II) and nickel (II) Adsorption Kinetics from Aqueous Metal Solutions Using Chemically Modified and uUnmodifiedaAgriculturalaAdsorbents. Afr J Biotechnol,. 2006;5(16):1475-1482.
- Huang X, Hu J, Qin F, et al. Heavy metal pollution and ecological assessment around the Jinsha coal-fired power plant (China). Int J Environ Res Public Health. 2017;14(12):1589. doi:10.3390/ijerph14121589.
CrossRef - Zhang Q, Ye J, Chen J, Xu H, Wang C, Zhao M. Risk assessment of polychlorinated biphenyls and heavy metals in soils of an abandoned e-waste site in China. Environ Pollut. 2014;185:258-265. doi:10.1016/j.envpol.2013.11.003.
CrossRef - McAuliffe ME, Williams PL, Korrick SA, Altshul LM, Perry MJ. Environmental exposure to polychlorinated biphenyls and p, p-DDE and sperm sex-chromosome disomy. Environ Health Perspect. 2012;120(4):535-540. doi:10.1289/ehp.1104017.
CrossRef - Meena BS, Meena M, Chandrawat U, Rani A. Dry deposition of heavy metals assoc its characterization in an industrial. Res J Recent. 2019;8(3):1-11.
- Meena M, Meena M, Chandrawat U, Rani A. Seasonal variations and sources of heavy metals in free fall dust in an industrial city of Western India. Iran J Energy Environ. 2014;5(2):160-166. doi:10.5829/idosi.ijee.2014.05.02.07.
CrossRef - Adamson IYR, Prieditis H, Hedgecock C, Vincent R. Zinc is the toxic factor in the lung response to an atmospheric particulate sample. Toxicol Appl Pharmacol,. 2000;166(2):111-119. doi:10.1006/taap.2000.8955.
CrossRef - Sun G, Crissman K, Norwood J, Richards J, Slade R, Hatch GE. Oxidative interactions of synthetic lung epithelial lining fluid with metal-containing particulate matter. Am J Physiol Lung Cell Mol Physiol. 2001;281(4):L807-L815. doi:10.1152/ajplung.2001.281.4.L807.
CrossRef - Rajput JS, Trivedi MK. Determination and assessment of elemental concentration in the atmospheric particulate matter: a comprehensive review. Environ Monit Assess. 2022;194(4):243. doi:10.1007/s10661-022-09833-9.
CrossRef - Araja A, Bertins M, Celma G, Busa L, Viksna A. Distribution of minor and major metallic elements in residential indoor dust: A case study in Latvia. Int J Environ Res Public Health. 2023;20(13):6207. Doi:10.3390/ijerph20136207.
CrossRef - Rashed MN. Total and extractable heavy metals in indoor, outdoor and street dust from Aswan City, Egypt. CLEAN Soil Air Water. 2008;36(10-11):850-857. doi:10.1002/clen.200800062.
CrossRef - Yaroshevsky AA. Abundances of chemical elements in the Earth’s crust. Geochem Int. 2006;44(1):48-55. doi:10.1134/S001670290601006X.
CrossRef - Nowrouzi M, Pourkhabbaz A. Application of geoaccumulation index and enrichment factor for assessing metal contamination in the sediments of Hara Biosphere Reserve, Iran. Chem Speciation Bioavailability. 2014;26(2):99-105. doi:10.3184/095422914X13951584546986
CrossRef - Ackermann F. A procedure for correcting the grain size effect in heavy metal analyses of estuarine and coastal sediments. Environ Technol Lett. 1980;1(11):518-527. doi:10.1080/09593338009384008
CrossRef - Shukla SP, Sharma M. Neutralization of rainwater acidity at Kanpur, India. Tellus. 2010;62(3):172-180. doi:10.1111/j.1600-0889.2010.00454.x.
CrossRef - Zhang P, Dudley N, Ure AM, Littlejohn D. Application of principal component analysis to the interpretation of rainwater compositional data. Anal Chim Acta. 1992;258(1):1-10. doi:10.1016/0003-2670(92)85192-9.
CrossRef - Meena M, Meena BS, Chandrawat U, Rani A. Seasonal variation of selected metals in particulate matter at an industrial city Kota, India. Aerosol Air Qual Res. 2016;16(4):990-999. doi:10.4209/aaqr.2015.02.0074.
CrossRef - Radhi AB, Shartooh SM, et al. Heety, E.A. 2021. Heavy Metal Pollution and Sources in Dust from Primary Schools and Kindergartens in Ramadi City, Iraq. Iraqi Journal of Science:1816-1828.
CrossRef - Tashakor M, Behrooz RD, Asvad SR, Kaskaoutis DG. Tracing of heavy metals embedded in indoor dust particles from the industrial City of Asaluyeh, south of Iran. Int J Environ Res Public Health. 2022;19(13):7905. doi:10.3390/ijerph19137905.
CrossRef - Wang F, Meng D, Li X, Tan J. Indoor-outdoor relationships of PM2. 5 in four residential dwellings in winter in the Yangtze River Delta, China. Environ Pollut. 2016;215:280-289. doi:10.1016/j.envpol.2016.05.023.
CrossRef - Liu R, Wang Q, Lu X, Fang F, Wang Y. Distribution and speciation of mercury in the peat bog of Xiaoxing'an Mountain, northeastern China. Environ Pollut. 2003;124(1):39-46. doi:10.1016/s0269-7491(02)00432-3.
CrossRef - Moja SJ, Mnisi JS. Seasonal variations in airborne heavy metals in Vanderbijl park, South Africa. J Environ Chem Ecotoxicol. 2013;5(9):227-233.
- Banerjee D. Study of precipitation chemistry over an industrial city. Int J Environ Sci Technol. 2008;5(3):331-338. doi:10.1007/BF03326028.
CrossRef - Krolak E. Heavy metals in falling dust in Eastern Mazowieckie Province. Pol J Environ Stud. 2000;9(6):517-522.
- Wang X, Sato T, Xing B, Tamamura S, Tao S. Source identification, size distribution and indicator screening of airborne trace metals in Kanazawa, Japan. J Aerosol Sci. 2005;36(2):197-210. doi:10.1016/j.jaerosci.2004.08.005.
CrossRef - Fang GC, Wu YS, Huang SH, Rau JY. Review of atmospheric metallic elements in Asia during 2000-2004. Atmos Environ. 2005;39(17):3003-3013. doi:10.1016/j.atmosenv.2005.01.042.
CrossRef - Banerjee AD. Heavy metal levels and solid phase speciation in street dusts of Delhi, India. Environ Pollut. 2003;123(1):95-105. doi:10.1016/s0269-7491(02)00337-8.
CrossRef - Gul HK, Gullu G, Babaei P, Nikravan A, Kurt-Karakus PB, Salihoglu G. Assessment of house dust trace elements and human exposure in Ankara, Turkey. Environ Sci Pollut Res Int. 2023;30(3):7718-7735. doi:10.1007/s11356-022-22700-x.
CrossRef - Monged MHE, Hassan HB, El-Sayed SA. Spatial distribution and ecological risk assessment of natural radionuclides and trace elements in agricultural soil of northeastern Nile valley, Egypt. Water Air Soil Pollut. 2020;231(7):338. doi:10.1007/s11270-020-04678-9.
CrossRef - Gope M, Masto RE, George J, Balachandran S. Tracing source, distribution and health risk of potentially harmful elements (PHEs) in street dust of Durgapur, India. Ecotoxicol Environ Saf. 2018;154:280-293. doi:10.1016/j.ecoenv.2018.02.042.
CrossRef - Khademi H, Gabarrón M, Abbaspour A, Martínez-Martínez S, Faz A, Acosta JA. Environmental impact assessment of industrial activities on heavy metals distribution in street dust and soil. Chemosphere. 2019;217:695-705. doi:10.1016/j.chemosphere.2018.11.045.
CrossRef - Araja A, Bertins M, Celma G, Busa L, Viksna A. Distribution of minor and major metallic elements in residential indoor dust: A case study in Latvia. Int J Environ Res Public Health. 2023;20(13):6207. Doi:10.3390/ijerph20136207.
CrossRef






