Recent progress in doped TiO2Photocatalysis and Hybrid Advanced Oxidation Processes for Organic Pollutant Removalfrom Wastewater
Corresponding author Email: darshana333@gmail.com
DOI: http://dx.doi.org/10.12944/CWE.17.1.13
Hybrid advanced oxidation processes (HAPOs) for the removal of non-biodegradable organics from wastewater have been studied in recent literature. With the increase in industrial development, the quantity of wastewater generated from these industries also organic wastewater produced by industrial manufacturing has posed threats to the environment.AOP’s are one of the promising advanced technologies for mineralization of organics present in wastewater. Hybrid advanced oxidation process based on the ozonation, sonolysis, Photo-Fenton reagents and electrochemical method, has greater potential for complete mineralization of recalcitrantorganics. This review article includes recent progress in the research and application of TiO2 photocatalysis for the removal of nonbiodegradable organic pollutants present in water. It will provide a quick reference for various hybrid AOPs systems and their effectiveness. This review article provides quick insights into (1) hybrid AOP for treatment of various industrial effluents or model effluents, (2) work done on doped/co-doped photocatalyst as heterogeneous catalysts (3) study of parameters affecting the photocatalysis to enhance complete oxidation of organics present in wastewater. A mechanistic investigation of hybrid advanced oxidation processes with combinations of sonolysis and Fenton process coupled with UV, adsorption and addition of biochar has been discussed.
Copy the following to cite this article:
Bhatti D. T, Parikh S. P. Recent progress in doped TiO2Photocatalysis and Hybrid Advanced Oxidation Processes for Organic Pollutant Removalfrom Wastewater. Curr World Environ 2022;17(1). DOI:http://dx.doi.org/10.12944/CWE.17.1.13
Copy the following to cite this URL:
Bhatti D. T, Parikh S. P. Recent progress in doped TiO2Photocatalysis and Hybrid Advanced Oxidation Processes for Organic Pollutant Removalfrom Wastewater. Curr World Environ 2022;17(1).
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 12-11-2021 |
|---|---|
| Accepted: | 22-02-2022 |
| Reviewed by: | 
 Kosar Hama Aziz
Kosar Hama Aziz
|
| Second Review by: |

 Dr. Jayvardhan Balkhande
Dr. Jayvardhan Balkhande
|
| Final Approval by: | Dr. Saravanan Pichiah |
Introduction
Innovations and productions of new medicines increased number of pharmaceutical industries with accumulation of waste in rivers and on land. Environmental management part always found non-focused and lead to degradation of nature. Researchers are working on these issues to resolve these problems.This situation enforced research towards zero effluent discharge, green technology and cleaner development mechanism. Semiconductor photocatalysis has been extensively studied by many researchers for the complete oxidation of refractory organics present in effluent1–3, water splitting for hydrogen production 4 and solar cells 5. The application of TiO2 as a photocatalyst is limited by UV radiations and recombination of the hole and electron pairs 6-7. Rapid industrialization has vastly increased water and air pollution problems as the current generation are interested more in profit and less concerned about waste generation. This situation demands fruitful research be done on waste minimization to avoid such situations and to achieve sustainable development. Objective of this review is to search for efficient and cost-effective AOP for wastewater treatment.Solar light-driven effluent treatment methods have been focused and developed for research 8. Titanium dioxide is an N-type semiconductor having an oxygen deficit in its structure. TiO2 is a superior, nontoxic stable and economical photocatalyst that provides a non-selective and efficient oxidizing agent, Hydroxyl radical (OH*) 9-10. TiO2 has shown certain limitations as a photocatalyst: 1) it has a large bandgap and works only under UV radiations; 2) its low quantum yield of OH* due to recombination of holes 11.
Metal doping in TiO21) improves its absorbance in the visible region, e.g. a Ag: 300-800 nm, Co: 400-650 nm and Fe: 300-800 nm, 12-14; and allow it to work under solar radiation to make cost-effective treatment.; 2) provides the excellent trap of electrons prevents recombination of e- and holes results in superior photoactivity 15; 3) the Bandgap reduces from pure TiO2 (3.1 eV) to doped TiO2 (2.8 eV) 16-17. Silver and iron are extensively investigated as a dopant for TiO2and proved superior photocatalysts for mineralization of active pharmaceutical ingredients(API) 18–20. Co-doping of TiO2 using metal dopants is a promising technologyfor solar mineralization of refractory organics in wastewater. Doping of TiO2 with Fe and Ag metals enhances the photocatalytic activity due to large reactive sites for photocatalysis 21–26. Nanomaterials have magical physical and ocular characteristics due to their size and incarceration e? to initiate quantum properties. Nanopowder absorbs much more solar radiation compared to nanofilms. Size, morphology and optical properties can be controlled during solar photocatalysis and photovoltaics results in better absorption of solar irradiations27, 28. Several studies on the photoactivity of Ag-doped TiO2 and Ag-Fe co-doped TiO2 (Ag-Fe CT) catalyst proved co-doped catalyst superior over undoped TiO2 25, 29, 30. Anisotropic structure of Ag dopant improved solar radiation absorbance 31. In this review, we have described recent progress in advanced oxidation processes with metal dopants, co-doped photocatalysts with their properties and bandgap. Synthesis of nano-doped TiO2, mechanism of degradation by photocatalysis, operating variables and their effects on degradation and different techniques to modify optical properties of TiO2 such as the use of metal and non-metal dopants, nanofilms, nanotubes and nanowires are discussed. The feasibility and the effectiveness of recycled photocatalyst have been studied. Hybrid AOPs is proved efficient compared to conventional AOP for complete mineralization of complex organics. Hybrid AOP using Fe doped TiO2 has shown dual characteristics of photocatalysis and Fenton reaction, which has improved decolorization of wastewater 32. Photocatalytic treatment work under normal ambient conditions 33. Efficient methylene blue degradation using combining AOP with Fenton reagents, results in production of more OH radicals 34. Diclofenac and ibuprofen were converted efficiently in to biodegradable intermediates using planar falling film reactor andCoated TiO2 on a Pilkington Active glass under UV radiations 35,36. This review will be useful to select efficient hybrid AOP for specific industrial wastewater treatment.
Advanced Oxidation Processes
AOPs are effluent treatment technology that produces a hydroxyl radical (OH) with highest oxidation potential and performs oxidation of organics to produce carbon dioxide and water as end products. These processes use ozone, photo Fenton reagents, hydrogen peroxide, or semiconductor photocatalysis to generate OH. TiO2 was focused on photocatalysis by many researchers. It is available in three forms anatase, brookite and rutile. Amongst all these, the tetragonal anatase structure performs efficient photocatalysis 37, 38.Various advanced oxidation processes consist of pollutant removal technologies in which hydrogen radicals serve as an active medium. The methods are separated according to the source of the formation of hydroxyl radicals as shown in Fig. 139.
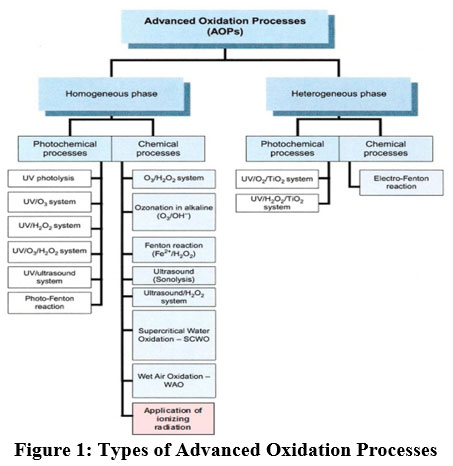 |
Figure 1: Types of Advanced Oxidation Processes. |
Table 1 shows the oxidation potentials of various oxidizing agents. OH. Radical is nontoxic, nonselective and hasthe highest oxidation potential hence it is capable to mineralize a major category of organic materials from wastewater during photocatalysis.
Table 1: Oxidation potential of different oxidants[40].
|
Oxidizing Agent |
Potential of oxidation (V) |
|
OH• |
2.8 |
|
O2- |
2.4 |
|
O3 |
2.1 |
|
H2O2 |
1.8 |
|
HOCl |
1.5 |
|
O2 |
1.2 |
Some benefits of research of AOPs are as follows:
Newer technology to produce strong and non-specific hydroxyl radical oxidizing agent;
To set up the highest standards for effluent treatment;
To develop an advanced mode of operation and competitiveness.
Table 2 summarizes different AOPs used for the degradation of various organics.Table 2: Different Advanced Oxidation Processes for component degradation.
|
Sr. No. |
AOPs |
Component for degradation |
Experimental conditions |
Results |
Ref. |
|
1 |
TiO2-photocatalytic degradation |
Tetracycline (TC) |
Total Carbon 5–20 mg/L, TiO2- 0.5-2 g/L 30 min in dark,2 hr for photocatalytic degradation, TiO2- 1 g/L, 12 W halogen lamp Total Carbon 10 mg/L |
Optimum TiO2 conc.1 g/L Toxicity removal 84 % in 240 min |
[19] |
|
2 |
aerobic, anaerobic, aerobic/anaerobic reactor, sonication, photocatalysis reactor |
Ciprofloxacin (CIP) |
Aerobic/anaerobic sequential reactor system – Hydraulic retention time=10 days Organic loading rate= 0.2 g COD/L, Sonication at a power of 640 W and 35 kHz 45°C,pH 7, 45 min irradiation time, 210 W UV lamp, 0.5 g/L TiO2 25°C |
COD removal and CIP yields were 95% and 83%, 95% and 81% after 45 min, 98% and 88% |
[41] |
|
3 |
TiO2-assisted ozonation in water |
cyanotoxin cylindrospe-rmopsin(CYN) |
pH 7, O30.25-2 mg/L, TiO2=500 mg/L, CYN 5 mg/L
|
Pseudo first order, ozonation increased degradation from 75.7% to 98.9%. |
[42] |
|
4 |
hybrid ozonation-nano filtration- continuous process |
|
ozone – 1.17-4.85 mg/lit, NF module.- (AFC30 ) Polyamide film membrane with 75% CaCl2 retention, Flow rate: 8 L/min, 30 bar, 25oC |
COD inlet 1300 mg/L COD outlet 50 mg/L (96.15 %) ozone treatment increase permeate flux and decreased fouling index due to less flocculation so pores are not clogged. |
[43] |
|
5 |
Ozonation, H2O2/UV and TiO2 Photocatalysis |
Carbamazepine, propranolol, clofibric acid, diclofenac, ofloxacin, sulfamethoxazole, blue-green algae |
Hydrogen peroxide (30% w/w), pH 7.6, time: 20 min , ozone 13.875 mg/L, 0.3 gm/L TiO2, UV 300 W, 48hr |
Complete removal of toxicity (% survival of blue-green algae Synechococcusleopoliensis, rotifer), 80 % removal of each organic |
[44] |
|
7 |
Combined GAC adsorption and UV254/H2O2
|
pharmaceutical wastewater |
2.12 to 6.37 mg H2O2/mgCOD, time 3hr, pH 3.4 20-60 min GAC, pH 3.4 |
Highest TOC removal 88% |
[45] |
Major merits of AOP includes the faster rate of mineralization, nonbiodegradable organics are completely oxidized into CO2 and H2O, treated effluent can be directly reused without further purification, avoid sludge generation and its handling problems, it can be easily clubbed with existing ETP with little modification, and economic operation and maintenance compared to incineration. Demerits of AOPs are higher capital costs, complex and unknown reaction chemistry may sometimes lead to more hazardous intermediates formation and photochemical reactor design and operationare difficult. Challenges of AOPs arePhotocatalyst deactivation and unknown routes for different reactions 46, development of proper doped catalysts to enhance the absorption of solar radiation, the selectivity of photocatalyst may sometimes pose a problem in treatment when a mixture of different organics is present, electron and hole recombine to result in lower net generation of OH radicals, scale-up and commercialization of process47 and UV radiations may sometimes degrade ozone, chlorine and hydrogen peroxide which are useful oxidizing agents in the process39.
Titanium Dioxide Photocatalysis
Semiconductor oxides have a greaternumber of surface atoms ona surface which enables photon absorption and performs various oxidation and reduction reactions for complete removal of a variety of organics from aqueous solutions. Titanium dioxide is widely preferred for photocatalysis due to its stability, reusability, nontoxicity, anti-corrosiveness and low cost. Different other oxides that can also be used for photocatalysis are zinc, tin, zirconium, cadmium and iron.Hydroxyl radicals react with organics to produce carbon dioxide and water 6,48. The main reactions involved in photocatalysis are shown below (equation (1) to equation (8)) 49 :
Photon absorption:
MO + h? → MO + e−CB+ h+VB (1)
Oxidation:
h+ + OH- (Surface) → OH • (2)
H2O + h+ → OH •+H+ (3)
H2O + h+ → H+ +½ H2O2 (4)
H2O2→ 2 OH• (5)
Reduction:
O2 +e−→ O2− (6)
H2O +O2− + H+ → H2O2 + O2 (7)
Electron and hole combination:
h+ + e−→ energy (8)
where MO is a metal oxide, h? are photons, h+ are holes. When photons bombard on TiO2 surface it enables electron movement and reactions on an interface where large numbers of organic substances are absorbed from the effluent. Semiconductor TiO2 absorbs photons and transferelectron from the valance band (vb) to the conduction band (cb). On the valence band, holes are generated which reacts with H2Oor OH- to produce hydroxyl radicals. TiO2 is N-type semiconductor material. Hole performs oxidation reactions and electron performs reduction reactions as shown in equations (1) to (9) on the surface along with complete oxidation of organics to produce CO2 and H2O.
 |
Figure 2: Mechanism of photocatalysis [50]. Click here to view Figure |
When semiconductors such as TiO2 absorb light e- jumps from the vb to the cb. Nanoparticles have a large surface to volume ratio and also contain more atoms on their surface which substantially absorb photons. Nanoparticles can perform photocatalysis rapidly before e- and hole recombine17, 51 Parameters affecting photocatalysis are Organic load, catalyst concentration, reactor design (batch, continuous, immobilized/suspended catalyst etc.), adsorption and UV irradiation time (optimum), temperature, pH, light intensity and presence of ionic species 81.
Doping in Nano-Structured TiO2 for enhanced photocatalytic activity
Doping is one of the methods to improve optical properties, reduce bandgap and overcome e-/hole recombination as metals trap e- result in enhanced photocatalytic activity of semiconductor oxides. Doping will provide efficient and economical photocatalysis as it can replace UV photocatalysis with solar or visible irradiations.Loading of TiO2 surface with dopant will engineer the photocatalyst with improved trapping of charge carriers. Thus Doping increases organics degradation efficiency 52. Dopant will create oxygen defects and shifts light absorption from UV to the visible region by improving absorption bandwidth. The efficiency of photocatalysis may differ based on the position ofthe dopant on the TiO2 structure. Based on synthesis methods, the dopant can take a position on the surface or it can be included in lattice structure or as core and thus these positions may lead to different photocatalytic activity and degradation efficiency. Metals and non-metals both can work as dopants but major research concludes that metal dopants possess strong surface plasmon resonance (SPR), work efficiently under solar radiations during photocatalysis 53.
For efficient photocatalysis, the bandgap should be lower which promotes the transfer of e- and holes. This will also influence the redox potential of photogenerated electrons and the oxidation potential of holes 53.The handling of TiO2 powder form is difficult and the cost of UV radiation makes the treatment energy-intensive and uneconomical. These issues limit the commercialization of AOPs for industrial effluent treatment. These limitations can be overcome by surface modification of TiO2 with transition metal doping which reduces the bandgap and greater absorption of visible light is possible, also the dopant metals trape e- and prevent its recombination with holes, hence, the photocatalysis can be performed under solar radiation to make system economical for removal of refractory organics compared to incineration treatment. Various metal dopants are Chromium, manganese, cobalt, copper, iron Nickle, Zinc, cerium, Neodymium, Eurotium, Lanthanum, etc. and various non-mental dopants are Palladium chloride, carbon, nitrogen, and Flouride.
Recyclability of Photocatalyst
TiO2 doped with 33% Fe2O3core-shell photocatalyst has enhanced paracetamol removal by photocatalysis from water and the photocatalyst could be easily separated and reused for four recycle runs [28]. Ag decorated Fe3O4/TiO2 coated cenosphere prepared via Modified sol-gel and wet impregnation can be recycled for 8 cycles with a slight reduction in Methylene blue degradation efficiency 26. The novel engineered photocomposite core-shell structure Fe3O4@SiO2@TiO2 showed greater photoactivity compared to commercial TiO2. The catalyst provided easy separability using a magnet and was recycled for 10 numbers of recycling runs without a decrease in efficiency [22]. When the Ag-Fe CT with Ti/Ag mole ratio 30 photocatalystswere reused for six numbers of runs, 63.25% COD was removed in 5 hr solar light irradiation, indicating more deactivation of the catalyst during photocatalysis; which represented that the Ag-Fe CT 30 could be recyclable effectively for 4 cycles. The reduction in % COD removal was only less than 5% after three runs of recycling for Ag-Fe CT 30. Ag-Fe CT 30 catalyst has proved its stability even after 4 recycle runs and it can perform photocatalysis under solar radiation effectively for the photocatalysis of drug intermediates 16. Dye degradation efficiency by Fe3+ doped TiO2has been found to decrease by 9% at the end of six recycle runs55. Ag-Fe CT and Fe2O3/SiO2 co-doped TiO2 and Ag-Fe CT supported on graphene oxide has shown good stability for 5 recycle runs[58].Table 4summarizes the literature review done for the recyclability of photocatalysts. The photocatalysts can be recovered after treatment and efficiently used for several runs without loss in efficiency of treatment or component degradation. The result showed a decrease in photocatalytic activity with an increase in the number of recycling runs as the poisoning of the catalyst increases due to surface blockage, less adsorption and low rate of oxidation reaction 7.
Table 4: Feasibility and effectiveness of photocatalyst for recyclability.
|
Sr. No. |
Catalyst |
Synthesis method |
Model pollutant and expt. Conditions |
Recycla-bility runs |
Result |
Ref. |
|
1 |
Fe3O4–TiO2 |
Solvothermal and micro-thermal method |
Phenol, UV light, 100-300 min, 0.5 g/L
|
2 |
Degradation was 100%, 70%, 32% for P25 and Fe3O4–TiO2 (3 ml titanium butoxide), Fe3O4–TiO2 (10 ml Titanium butoxide) respectively |
[2] |
|
2 |
Fe3O4@SiO2/β-NaYF4:Yb3+,Tm3+/TiO2 |
sol– gel process and solvo-thermal |
methylene blue, methyl orange, rhodamine B, and phenol under, 1-10 ppm, 144 min, Laser light, 10 g/L |
4 |
76.62%, 68.48%, 30.05% and 27.16% |
[66] |
|
3 |
Ag-doped TiO2, Ag:Ti molar ratio: 0.02-0.12
|
solgel |
Acetamiprid- 20 mg/L-insecticide, UV light, 60 min, 0.4 g/L |
6 |
Ag/Ti = 0.06 opti, as Ag increase rutile phase increase |
[88] |
|
4 |
Fe3+-doped TiO2-1-4 wt % |
modified sol-gel |
azo dye acid orange 7-50 mg/L, solar, UV and visible light, 18 min, 0.3 g/L |
4 |
100 % UV, 100 % visible, 90 % solar in 2 hr, 3 wt % opt-98.9 % |
[55] |
|
5 |
N-TiO2/Fe3O4@SiO2 and Ag-Fe |
coprecipitation |
bisphenol A: 2 mg/L, visible light, 90 min |
3 |
100 % and 88% using Ag-Fe and N-TiO2 /Fe3O4 @SiO2 respectively |
[58] |
|
6 |
Ag-doped TiO2-P25 supported on Clay beads, Fe-Ag-TiO2 composite (1.5 wt %) |
surface impregnation method |
Drug: pentoxifylline (PEN) 50 mg/L, 40 ml solution, solar, 1.5 g/L, 30 min |
10 |
Ag-TiO2-P25: Opt.: 0.75 g/L cat conc., 75% and 68% degradation in TOC and COD resp. 90% degradation of PEN in 30 min |
[30] |
|
7 |
graphene oxide supported Ag-Fe TiO2 -1 wt% of Ag |
chemical reduction and the hydrothermal |
methylene blue 20 mg/L and 4-NP, visible, 150 min, 0.2 g/L |
3 |
rGO supported Ag-Fe CT, rGO supported Fe -TiO2, Fe - TiO2 and undoped TiO2-95 and 89%, 82%, and 74.6%, respectively |
[29] |
|
8 |
Clay suppo. Fe doped TiO2 (1-4 %: 2% opt) |
surface impregnation method |
Pesticide-Carbendazim: 4-10 gm/L, UV and solar, 4 g/50 clay beads, 300 min |
40 |
70 % degrade-UV. TiO2: 82 UV+63 sun light, Fe TiO2- 93 % sun light and 67 % UV |
[89] |
|
9 |
Fe3+ doped TiO2 film- with Fe3+ =0, 1, 3, 5, 7 and 10 |
spin coating |
methylene blue, 5 mg/L, 25 mL, visible, 240 min |
10 |
96.7 % at 7% opt. 83.5 % at 10th round end |
[90] |
|
10 |
Fe doped TiO2-3% |
Sol gel |
methylene blue: 10-5 mg/L visible, 150 min, 0.5 g/L |
|
59, 97, 79 % for TiO2, 3% Fe and 7% Fe-TiO2 |
[7] |
|
11 |
Cu2+, Ag+, Zn2+, Fe3+, and Al3+ ion and Pt metallic +effect of doping, Cr3+, Mn2+ and Co2+: -ve effect of doping
|
Sol gel, 0.5 mol % dopant metal |
Para nitrophenol: 10-4 mol/L, 480 min, 1 g/L |
3 |
50 % -TiO2, 55: Fe 0.5, Fe 2 : 35, Fe 5: 15, Ag 0.5: 58, Ag 2: 60, Pt 0.1: 79 % |
[91] |
|
12 |
Ag-doped TiO2 pillars-2.8 % |
Wet impregnation and high temp thermal reduction |
2,4-dichlorophenol -5 mg/L-30 ml, visible, 120 min 1.67 g/L |
10 |
99 % |
[92] |
|
13 |
Au-Ag NPs-decorated TiO2-modified Fe3O4 |
Solvo thermal |
Textile waste water- Rh6G dye 30 ppm, xenon lamp, 60 min 2.67 g/L |
5 |
95 % removal. 8% efficiency decreased after 5 runs |
[38] |
*NA: data not available
Ammonical nitrogen removal using photocatalysis
NH4-N removal is higher in alkaline pH during photocatalysis. At lower pH, the surface of photocatalyst has a positive charge whereas ammoniacal nitrogen compounds can be adsorbed only on the surface which has a negative charge [93].NH4-N removal is more when pH is greater than 10. Researchers have reported that it is not possible to oxidize NH4-N OH by radicals [94]. When pH is above 9, NH4-N can be converted into NH3 95. Hence acidic or neutral condition does not favor NH3 –N removal simultaneously with organics. Table 5summariesresearch done for ammonical nitrogen removal by photocatalysis.
Table 5: Ammonical nitrogen removal during photocatalysis.
|
Sr. No. |
Catalyst |
Synthesis method |
Model pollutant |
light |
OptpH |
Catalyst dose |
Time, hr |
Result |
Ref. |
|
1 |
TiO2 film on glass beads: to 10 layers of TiO2 thin film. |
Coating with sol-gel method |
NH4Cl solution 300 ml, ammonia conc. 700 mg/L |
UV light |
7 |
film |
2 hr |
6 coating opt, 70 % removal efficiency |
[96] |
|
2 |
Cu/ZnO/rGO Nanocomposite |
Sol-gel |
Domestic wastewater NH4+-N: 10, 30, 50, 70, and 100 mg/L |
Visible-Xenon lamp |
10 |
0.2-2 g/L, opt 2 |
2 hr |
Optimum : NH4+con.= 50 mg/L, catalyst conc.= 2 g/L, pH 10. 83% removalefficiency
|
[56] |
|
3 |
La/Fe/TiO2 composite |
Sol-gel |
NA |
500 W mercury lamp. |
10 |
1 g/L, |
3 hr |
64.6% removal efficiency |
[97] |
|
4 |
TiO2 |
Sol-gel |
Secondary treated effluent: Ammonia conc. 26 – 214 mg/l |
UV light |
10.7 |
2.1 g/L |
3.5 hr |
50 % removal efficiency |
[95] |
|
5 |
Ag/ Fe co-doped TiO2 |
Sol-gel |
Industrial effluent, COD: 88660 mg/L, NH3-N:3287 mg/L |
Solar |
5 |
1g/L |
5 hr |
64.69%% COD removal, 16.05% NH3-N removal |
[16] |
*NA: data not available
Hybrid Advanced Oxidation processes
COD removal using three methods, combining electrochemical process with AOP, Fenton reagent and flotation HAOP technology has been proved effective in the treatment of pharmaceutical wastewater for COD removal [98]. An ultrasound when used in combination with photocatalysis, Fenton Reagent and the Photolysis process, proved efficient for non-biodegradable toxic organics removal. This combination of AOP will overcome problems of repelling photocatalyst and pollutants due to similar charges. A sonophotocatalysis has been found effective for the removal of variety of organics present in wastewater [99]. Hybrid AOPs with sonolysis, Fenton and photo– ferrioxalate system with sonolysis has been studied for degradation of two dyes: Acid Red B and Methylene Blue. Sonolysis alone has shown the lowest efficiency. Coupling of sonolysis with either Fenton or photo- ferrioxalate system has shown the greater ability of decolorization. Ternary coupling of all these three systems has shown a negative effect of dyes degradation due to the interaction of individual mechanisms 100.
Table 6: Hybrid Advanced Oxidation processes
|
Sr. No. |
Hybrid AOP |
Compound for degradation/treatment |
Experimental condition |
Result |
Ref. |
|
1 |
Advanced oxidation with O3 addition, adsorption by activated charcoal
|
Pharmaceutical effluent |
pH 5-11,, time – AOP- 3 hr, adsorption with charcoal- 2.5 hr |
H2O2 addition with AOP: COD removal: 75-88%. Further continuation of treatment with adsorption by activated charcoal- COD removal reached up to 93% |
[101] |
|
2 |
hydrodynamic cavitation with Fe3O4 nanophotocatalyst |
P-nitrophenol (PNP) |
8 atm -pressure, 3-pH, 20 mg/L-PNP, Fe3O4 to H2O2 ratio= 1:1, H2O2:0.6 mol/L, |
PNP degradation 78% |
[102] |
|
3 |
hydrodynamic cavitation (HC) with ZnO/ZnFe2O4 and persulfate system+ Magnetic separation for recycle |
Carbamazepine (CBZ) |
9 atm-pressure, 4-pH, 15 mg/L-CRZ, 18 W UV, 500 mg/L-Na2S2O8, 500 mg/L-ZnO/ZnFe2O4 |
98 % CBZ degradation |
[103] |
|
4 |
electrocatalytic process |
Industrial raw effluent (antibiotics) |
Cathod: carbon, anode: Ti/PtIr plate |
100% COD removal |
[33] |
|
5 |
UV/ZnOnps/O3 |
4-Nitro aniline (4-NA) |
catalyst dose: 3?g/L, pH:5, 4-NA: 10 mg/L, time: 60 min |
Degradation of 4-NA: 92% |
[104] |
|
6 |
MOFs@COFs hybrid materials with C3N4 : sulfate radical-based advanced oxidation processes |
bisphenol A (BPA)
|
Visible light |
BPA degradation 99% |
[105] |
|
7 |
UV-C or hydrogen peroxide |
Boscalid, pyraclostrobin, fenbuconazole and glyphosate-Pesticides removal on apple |
H2O2, UV-C |
glyphosate -99% removal, boscalid, pyraclostrobin and fenbuconazole degradation 88 %, 100 % and 70 % respectively |
[106] |
|
8
|
CuO particle-WO3 nanofiber hybrids-(adsorbent/photocatalyst) |
dyes |
WO3 nanofibers and CuOnps, visible light |
dyes removal-90%, 0.75 wt.% CuO adsorbed 38% higher and degraded 26% more methylene blue than WO3 nanofibers |
[107]
|
|
9 |
hybrid photocatalysis and Cr(III) dispersed membrane-geo polymer membrane separation |
Dyes wastewater |
50 min at 0.09 MPa |
100% degradation |
[108] |
|
10 |
nano-sheet C3N4-WO3 composite (nsCW21 with the addition of H2O2 |
Natural organic matter (NOM) |
5 hr, visible light photocatalysis |
Without the addition of H2O2: 71% removal, With the addition of H2O2: 91% removal, catalyst was stable up to 5 recycle runs. |
[109] |
|
11 |
Hybrid biochar-TiO2 |
textile wastewater treatment |
74.3?mg/g, biochar (30.4?mg/g) and pure TiO2 (1.50?mg/g) |
biochar and TiO2 alone - 85 % and 43 % degradation efficiencies respectively, coupling both-99% photo degradation efficiency |
[110] |
Conclusion
This review described various advanced oxidation processes with their merits, demerits, benefits and challenges. Various dopants have been compared for their enhanced photoactivity. The mechanism TiO2 semiconductor doped with Ag and Fe has been discussed. The degradation of various chemical compounds using TiO2-based photocatalysts, including mechanisms and factors affecting the process have been summarized. Hybrid AOP with photocatalyst is proved aneffective method for treatment of wastewater. Addition of different oxidizing agent and materials such as H2O2, Fenton reagents and biochar have increased organics removal efficiency from wastewater. Electro Fenton and electrolysis, cavitation was used effectively for wastewater treatment. Advanced oxidation with O3 addition, adsorption by activated charcoalfor pharmaceutical wastewater treatment was also effective. This paper concludes that proper selection of Hybrid AOPcan provide efficient mineralization of organics present in wastewater at low cost. Recyclability studies showed that photocatalyst can be separated after treatment and reused up to several runs efficiently without much decline in treatment efficiency.
Acknowledgement
The authors are grateful to VVP Engineering College, Rajkot for his support to carry out this critical review.
Conflict of interest
The authors do not have any conflict of interest.
Funding source
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- M. Makeswari and P. Saraswathi, “Photo catalytic degradation of methylene blue and methyl orange from aqueous solution using solar light onto chitosan bi-metal oxide composite,” SN Appl. Sci., vol. 2, no. 3, Mar. 2020, doi: 10.1007/s42452-020-1980-4.
CrossRef - Z. Lendzion-Bielu?, A. Wojciechowska, J. Grzechulska-Damszel, U. Narkiewicz, Z. ?niadecki, and B. Idzikowski, “Effective processes of phenol degradation on Fe3O4–TiO2 nanostructured magnetic photocatalyst,” J. Phys. Chem. Solids, vol. 136, Jan. 2020, doi: 10.1016/j.jpcs.2019.109178.
CrossRef - R. S. Dubey, K. V. Krishnamurthy, and S. Singh, “Experimental studies of TiO2 nanoparticles synthesized by sol-gel and solvothermal routes for DSSCs application,” Results Phys., vol. 14, p. 102390, Sep. 2019, doi: 10.1016/j.rinp.2019.102390.
CrossRef - S. Cao et al., “Photocatalytic pure water splitting with high efficiency and value by Pt/porous brookite TiO2 nanoflutes,” Nano Energy, p. 104287, Nov. 2019, doi: 10.1016/j.nanoen.2019.104287.
CrossRef - M. Iqbal et al., “Synthesis and characterization of transition metals doped CuO nanostructure and their application in hybrid bulk heterojunction solar cells,” SN Appl. Sci., vol. 1, no. 6, pp. 1–8, Jun. 2019, doi: 10.1007/s42452-019-0663-
CrossRef - P. S. Basavarajappa, S. B. Patil, N. Ganganagappa, K. R. Reddy, A. V. Raghu, and C. V. Reddy, “Recent progress in metal-doped TiO2, non-metal doped/codoped TiO2 and TiO2 nanostructured hybrids for enhanced photocatalysis,” Int. J. Hydrogen Energy, vol. 45, no. 13, pp. 7764–7778, Mar. 2020, doi: 10.1016/j.ijhydene.2019.07.241.
CrossRef - D. Komaraiah, E. Radha, N. Kalarikkal, J. Sivakumar, M. V. Ramana Reddy, and R. Sayanna, “Structural, optical and photoluminescence studies of sol-gel synthesized pure and iron doped TiO2 photocatalysts,” Ceram. Int., vol. 45, no. 18, pp. 25060–25068, Dec. 2019, doi: 10.1016/j.ceramint.2019.03.170.
CrossRef - A. Khanna and V. K. Shetty, “Solar light induced photocatalytic degradation of Reactive Blue 220 (RB-220) dye with highly efficient Ag@TiO2 core-shell nanoparticles: A comparison with UV photocatalysis,” Sol. Energy, vol. 99, pp. 67–76, Jan. 2014, doi: 10.1016/j.solener.2013.10.032.
CrossRef - D. Chen and A. K. Ray, “Photocatalytic kinetics of phenol and its derivatives over UV irradiated TiO 2,” 199 doi: 10.1016/S0926-3373(99)00068-5.
CrossRef - K. M. S. H. M. Nagaveni, “Photocatalytic degradation of various dyes by combustion synthesized nano anatase TiO2,” Appl. Catal. B Environ., vol. 45, no. 1, pp. 23–28, 2003, doi: DOI: 1016/S0926-3373(03)00124-3.
CrossRef - O. A. Al-Hartomy, “Synthesis, characterization, photocatalytic and photovoltaic performance of Ag-doped TiO2 loaded on the Pt-carbon spheres,” Mater. Sci. Semicond. Process., vol. 27, no. 1, pp. 71–78, 2014, doi: 10.1016/j.mssp.2014.06.025.
CrossRef - K. Varadharajan, B. Singaram, R. Mani, and J. Jeyaram, “Enhanced Visible Light Photocatalytic Activity of Ag and Zn Doped and Codoped TiO2Nanoparticles,” J. Clust. Sci., vol. 27, no. 5, pp. 1815–1829, 2016, doi: 10.1007/s10876-016-1044-5.
CrossRef - N. C. Birben et al., “Application of Fe-doped TiO2 specimens for the solar photocatalytic degradation of humic acid,” Catal. Today, vol. 281, pp. 78–84, Mar. 2017, doi: 10.1016/j.cattod.2016.06.020.
CrossRef - M. Cri?an et al., “The effects of Fe, Co and Ni dopants on TiO2 structure of sol–gel nanopowders used as photocatalysts for environmental protection: A comparative study,” Ceram. Int., vol. 42, no. 2, pp. 3088–3095, 2016, doi: 10.1016/j.ceramint.2015.10.097.
CrossRef - G. Ba?aran Dinda?, Y. Çali?kan, E. E. Çelebi, M. Tekba?, N. Bekta?, and H. C. Yatmaz, “Treatment of pharmaceutical wastewater by combination of electrocoagulation, electro-fenton and photocatalytic oxidation processes,” J. Environ. Chem. Eng., vol. 8, no. 3, Jun. 2020, doi: 10.1016/J.JECE.2020.103777.
CrossRef - D. T. Bhatti and S. P. Parikh, “Solar Light Induced Photocatalysis for Treatment of High COD Pharmaceutical Effluent with Recyclable Ag-Fe Codoped TiO2: Kinetics of COD Removal,” Curr. World Environ., vol. 15, no. 1, pp. 137–150, Apr. 2020, doi: 10.12944/cwe.15.1.17.
CrossRef - C. F. Carbuloni et al., “Degradation of metformin in water by TiO2–ZrO2 photocatalysis,” J. Environ. Manage., vol. 262, p. 110347, May 2020, doi: 10.1016/j.jenvman.2020.110347.
CrossRef - B. Darshana, S. Parikh, and M. Shah, “Potential of Ag–Fe co-doped TiO2 nanocomposite for solar photocatalysis of high COD pharmaceutical effluent and influencing factors,” Energy, Ecol. Environ., vol. 5, no. 5, pp. 344–358, Oct. 2020, doi: 10.1007/s40974-020-00162-6.
CrossRef - A. J. Watkinson, E. J. Murby, and S. D. Costanzo, “Removal of antibiotics in conventional and advanced wastewater treatment: Implications for environmental discharge and wastewater recycling,” Water Res., vol. 41, no. 18, pp. 4164–4176, Oct. 2007, doi: 10.1016/j.watres.2007.04.005.
CrossRef - X. D. Zhu, Y. J. Wang, R. J. Sun, and D. M. Zhou, “Photocatalytic degradation of tetracycline in aqueous solution by nanosized TiO2,” Chemosphere, vol. 92, no. 8, pp. 925–932, Aug. 2013, doi: 10.1016/j.chemosphere.2013.02.066.
CrossRef - W. Wang, J. Zhang, F. Chen, D. He, and M. Anpo, “Preparation and photocatalytic properties of Fe3+-doped Ag@TiO2 core-shell nanoparticles,” J. Colloid Interface Sci., vol. 323, no. 1, pp. 182–186, Jul. 2008, doi: 10.1016/j.jcis.2008.03.043.
CrossRef - Y. Chi et al., “Magnetically separable Fe3O4@SiO2@TiO2-Ag microspheres with well-designed nanostructure and enhanced photocatalytic activity,” J. Hazard. Mater., vol. 262, pp. 404–411, Nov. 2013, doi: 10.1016/j.jhazmat.2013.08.077.
CrossRef - T. Harifi and M. Montazer, “Fe 3+?:Ag/TiO 2 nanocomposite: Synthesis, characterization and photocatalytic activity under UV and visible light irradiation,” Appl. Catal. A Gen., vol. 473, pp. 104–115, Mar. 2014, doi: 10.1016/j.apcata.2014.01.005.
CrossRef - K. Tedsree, N. Temnuch, N. Sriplai, and S. Pinitsoontorn, “Ag modified Fe3O4@TiO2 magnetic core-shell nanocomposites for photocatalytic degradation of methylene blue,” in Materials Today: Proceedings, 2017, vol. 4, no. 5, pp. 6576–6584, doi: 10.1016/j.matpr.2017.06.170.
CrossRef - F. Petronella et al., “Multifunctional TiO2/FexOy/Ag based nanocrystalline heterostructures for photocatalytic degradation of a recalcitrant pollutant,” Catal. Today, vol. 284, pp. 100–106, 2017, doi: 10.1016/j.cattod.2016.11.0
CrossRef - J. Zhan, H. Zhang, and G. Zhu, “Magnetic photocatalysts of cenospheres coated with Fe3O 4/TiO2 core/shell nanoparticles decorated with Ag nanopartilces,” Ceram. Int., vol. 40, no. 6, pp. 8547–8559, 2014, doi: 10.1016/j.ceramint.2014.01.069.
CrossRef - L. L. Qian, Z. X. Wang, L. M. Zhu, K. Li, B. L. Li, and B. Wu, “Synthesis, structure, spectral characteristic and photocatalytic degradation of organic dyes of a copper metal-organic framework based on tri(triazole) and pimelate,” Spectrochim. Acta - Part A Mol. Biomol. Spectrosc., vol. 214, pp. 372–377, May 2019, doi: 10.1016/j.saa.2019.02.059.
CrossRef - N. Nasralla et al., “Structural and spectroscopic study of Fe-doped TiO2 nanoparticles prepared by sol-gel method,” Sci. Iran., vol. 20, no. 3, pp. 1018–1022, 2013, doi: 10.1016/j.scient.2013.05.017.
- D. P. Jaihindh, C. C. Chen, and Y. P. Fu, “Reduced graphene oxide-supported Ag-loaded Fe-doped TiO2 for the degradation mechanism of methylene blue and its electrochemical properties,” RSC Adv., vol. 8, no. 12, pp. 6488–6501, 2018, doi: 10.1039/c7ra13418e.
CrossRef - P. Bansal and A. Verma, “Applications of sunlight responsive Fe-Ag-TiO2 composite incorporating in-situ dual effect for the degradation of pentoxifylline,” Mater. Sci. Eng. B Solid-State Mater. Adv. Technol., vol. 236–237, pp. 197–207, Oct. 2018, doi: 10.1016/j.mseb.2018.11.016.
CrossRef - O. D. Miller et al., “Fundamental limits to extinction by metallic nanoparticles,” Phys. Rev. Lett., vol. 112, no. 12, Dec. 2013, doi: 10.1103/PhysRevLett.112.123903.
CrossRef - B. Bethi et al., “Investigation of TiO2 photocatalyst performance for decolorization in the presence of hydrodynamic cavitation as hybrid AOP,” Ultrason. Sonochem., vol. 28, pp. 150–160, Jan. 2016, doi: 10.1016/J.ULTSONCH.2015.07.008.
CrossRef - A. Mukimin, H. Vistanty, and N. Zen, “Hybrid advanced oxidation process (HAOP) as highly efficient and powerful treatment for complete demineralization of antibiotics,” Sep. Purif. Technol., vol. 241, p. 116728, Jun. 2020, doi: 10.1016/j.seppur.2020.116728.
CrossRef - M. A. H. Karim et al., “Degradation of aqueous organic dye pollutants by heterogeneous photo-assisted Fenton-like process using natural mineral activator: Parameter optimization and degradation kinetics,” IOP Conf. Ser. Earth Environ. Sci., vol. 958, no. 1, p. 012011, Dec. 2021, doi: 10.1088/1755-1315/958/1/012011.
CrossRef - K. H. Hama Aziz, “Application of different advanced oxidation processes for the removal of chloroacetic acids using a planar falling film reactor.,” undefined, vol. 228, pp. 377–383, Aug. 2019, doi: 10.1016/J.CHEMOSPHERE.2019.04.160.
CrossRef - K. H. H. Aziz, K. M. Omer, A. Mahyar, H. Miessner, S. Mueller, and D. Moeller, “Application of Photocatalytic Falling Film Reactor to Elucidate the Degradation Pathways of Pharmaceutical Diclofenac and Ibuprofen in Aqueous Solutions,” Coatings 2019, Vol. 9, Page 465, vol. 9, no. 8, p. 465, Jul. 2019, doi: 10.3390/COATINGS9080465.
CrossRef - A. Giampiccolo et al., “Sol gel graphene/TiO2 nanoparticles for the photocatalytic-assisted sensing and abatement of NO2,” Appl. Catal. B Environ., vol. 243, pp. 183–194, Apr. 2019, doi: 10.1016/j.apcatb.2018.10.032.
CrossRef - M. Amoli-Diva, A. Anvari, and R. Sadighi-Bonabi, “Synthesis of magneto-plasmonic Au-Ag NPs-decorated TiO2-modified Fe3O4 nanocomposite with enhanced laser/solar-driven photocatalytic activity for degradation of dye pollutant in textile wastewater,” Ceram. Int., vol. 45, no. 14, pp. 17837–17846, Oct. 2019, doi: 10.1016/j.ceramint.2019.05.355.
CrossRef - M. Trojanowicz, A. Bojanowska-Czajka, I. Bartosiewicz, and K. Kulisa, “Advanced Oxidation/Reduction Processes treatment for aqueous perfluorooctanoate (PFOA) and perfluorooctanesulfonate (PFOS) – A review of recent advances,” Chemical Engineering Journal, vol. 336. Elsevier B.V., pp. 170–199, Mar. 2018, doi: 10.1016/j.cej.2017.10.153.
CrossRef - jayesh ruparelia Sharma Sandip, “A general review on Advanced Oxidation Processes for waste water treatment,” Chemistry (Easton)., 2011.
- C. Angela, Szabolcs, Zsuzsanna, Gábor, “Advanced Treatment of Pharmaceutical Wastewater by Nano Filtration and Ozonation,” 2012.
- C. C. Wu, W. J. Huang, and B. H. Ji, “Degradation of cyanotoxin cylindrospermopsin by TiO2-assisted ozonation in water,” J. Environ. Sci. Heal. - Part A Toxic/Hazardous Subst. Environ. Eng., vol. 50, no. 11, pp. 1116–1126, Sep. 2015, doi: 10.1080/10934529.2015.1047664.
CrossRef - G. Chittala and P. S. Mogadati, “PERFORMANCE STUDIES ON A PHARMACEUTICAL WASTEWATER TREATMENT PLANT WITH A SPECIAL REFERENCE TO TOTAL DISSOLVED SOLIDS REMOVAL,” Int. J. life Sci. Biotechnol. pharma Res., vol. 1, no. 1, 2012.
- R. Andreozzi et al., “Effects of advanced oxidation processes (AOPs) on the toxicity of a mixture of pharmaceuticals,” Water Sci. Technol., vol. 50, no. 5, pp. 23–28, 2004, doi: 10.2166/wst.2004.0304.
CrossRef - S. Ghafoori, K. K. Shah, M. Mehrvar, and P. K. Chan, “Pharmaceutical wastewater treatment using granular activated carbon and UV/H2O2 processes: Experimental analysis and modelling,” Can. J. Chem. Eng., vol. 92, no. 7, pp. 1163–1173, Jul. 2014, doi: 10.1002/cjce.21981.
CrossRef - Q. Chen et al., “Enhancing the photocatalytic and antibacterial property of polyvinylidene fluoride membrane by blending Ag–TiO2 nanocomposites,” J. Mater. Sci. Mater. Electron., vol. 28, no. 4, pp. 3865–3874, Feb. 2017, doi: 10.1007/s10854-016-5999-7.
CrossRef - Z. Xiu et al., “Recent advances in Ti3+ self-doped nanostructured TiO2 visible light photocatalysts for environmental and energy applications,” Chemical Engineering Journal. Elsevier B.V., Feb. 2019, doi: 10.1016/j.cej.2019.123011.
CrossRef - M. Huang et al., “Construction of g-C3N4 based heterojunction photocatalyst by coupling TiO2-SnO2 solid solution for efficient multipurpose photocatalysis,” J. Alloys Compd., p. 158132, Nov. 2020, doi: 10.1016/j.jallcom.2020.158132.
CrossRef - A. Khanna and V. Shetty K, “Solar light-driven photocatalytic degradation of Anthraquinone dye-contaminated water by engineered Ag@TiO2 core–shell nanoparticles,” Desalin. Water Treat., vol. 10, no. 3, pp. 376–385, 2014, doi: 10.1080/19443994.2014.888681.
CrossRef - H. Yang, K. Zhang, R. Shi, X. Li, X. Dong, and Y. Yu, “Sol-gel synthesis of TiO2 nanoparticles and photocatalytic degradation of methyl orange in aqueous TiO2 suspensions,” J. Alloys Compd., vol. 413, no. 1–2, pp. 302–306, Mar. 2006, doi: 10.1016/j.jallcom.2005.06.061.
CrossRef - M. Rabbani, S. Safalou moghaddam, and R. Rahimi, “Photocatalytic degradation of 4-nitrophenol in aqueous N, S-codoped TiO2 suspensions,” in international electronic conference on synthetic organic chemistry, Mar. 2019, pp. 1–30, doi: 10.3390/ecsoc-15-00791.
CrossRef - A. N. Banerjee, “The design, fabrication, and photocatalytic utility of nanostructured semiconductors: Focus on TiO2-based nanostructures,” Nanotechnology, Science and Applications, vol. 4, no. 1. pp. 35–65, Feb. 2011, doi: 10.2147/NSA.S9040.
CrossRef - S. M. Gupta and M. Tripathi, “A review of TiO2 nanoparticles,” Chinese Science Bulletin, vol. 56, no. 16. Springer, pp. 1639–1657, Jun. 2011, doi: 10.1007/s11434-011-4476-1.
CrossRef - M. Yeganeh et al., “The magnetic characterization of Fe doped TiO2semiconducting oxide nanoparticles synthesized by sol–gel method,” Phys. B Condens. Matter, vol. 511, pp. 89–98, Apr. 2017, doi: 10.1016/j.physb.2017.02.010.
CrossRef - F. Han, V. S. R. Kambala, R. Dharmarajan, Y. Liu, and R. Naidu, “Photocatalytic degradation of azo dye acid orange 7 using different light sources over Fe3+-doped TiO2 nanocatalysts,” Environ. Technol. Innov., vol. 12, pp. 27–42, Nov. 2018, doi: 10.1016/j.eti.2018.07.004.
CrossRef - S. He et al., “High efficient visible-light photocatalytic performance of Cu/ZnO/rGO nanocomposite for decomposing of aqueous ammonia and treatment of domestic wastewater,” Front. Chem., vol. 6, no. JUN, 2018, doi: 10.3389/fchem.2018.00219.
CrossRef - P. Wang, Y. Tang, Z. Dong, Z. Chen, and T. T. Lim, “Ag-AgBr/TiO2/RGO nanocomposite for visible-light photocatalytic degradation of penicillin G,” J. Mater. Chem. A, vol. 1, no. 15, pp. 4718–4727, Apr. 2013, doi: 10.1039/c3ta01042b.
CrossRef - J. He, X. Zeng, S. Lan, and I. M. C. Lo, “Reusable magnetic Ag/Fe, N-TiO2/Fe3O4@SiO2 composite for simultaneous photocatalytic disinfection of E. coli and degradation of bisphenol A in sewage under visible light,” Chemosphere, vol. 217, pp. 869–878, Feb. 2019, doi: 10.1016/j.chemosphere.2018.11.072.
CrossRef - M. F. Abdel Messih, M. A. Ahmed, A. Soltan, and S. S. Anis, “Facile approach for homogeneous dispersion of metallic silver nanoparticles on the surface of mesoporous titania for photocatalytic degradation of methylene blue and indigo carmine dyes,” J. Photochem. Photobiol. A Chem., vol. 335, pp. 40–51, Feb. 2017, doi: 10.1016/j.jphotochem.2016.11.001.
CrossRef - J. H. Kau, D. S. Sun, H. H. Huang, M. S. Wong, H. C. Lin, and H. H. Chang, “Role of visible light-activated photocatalyst on the reduction of anthrax spore-induced mortality in mice,” PLoS One, vol. 4, no. 1, Jan. 2009, doi: 10.1371/journal.pone.0004167.
CrossRef - † Zhibo Zhang, ‡ Chen-Chi Wang, ‡ and Rama Zakaria, and ‡ Jackie Y. Ying*, “Role of Particle Size in Nanocrystalline TiO2-Based Photocatalysts,” 1998, doi: 10.1021/JP982948+.
CrossRef - R. Vijayalakshmi and V. Rajendran, “Synthesis and characterization of nano-TiO 2 via different methods.” [Online]. Available: www.scholarsresearchlibrary.com.
- B. Cui, H. Peng, H. Xia, X. Guo, and H. Guo, “Magnetically recoverable core-shell nanocomposites γ-Fe 2O3@SiO2@TiO2-Ag with enhanced photocatalytic activity and antibacterial activity,” Sep. Purif. Technol., vol. 103, pp. 251–257, 2013, doi: 10.1016/j.seppur.2012.10.008.
CrossRef - N. I. Mohd Razip, K. M. Lee, C. W. Lai, and B. H. Ong, “Recoverability of Fe 3 O 4 /TiO 2 nanocatalyst in methyl orange degradation,” Mater. Res. Express, vol. 6, no. 7, Apr. 2019, doi: 10.1088/2053-1591/ab176e.
CrossRef - J. He et al., “Facile Formation of Anatase/Rutile TiO2 Nanocomposites with Enhanced Photocatalytic Activity,” Molecules, vol. 24, no. 16, p. 2996, Aug. 2019, doi: 10.3390/molecules24162996.
CrossRef - Z. Chen and M. L. Fu, “Recyclable magnetic Fe3O4@SiO2/β-NaYF4:Yb3+,Tm3+/TiO2 composites with NIR enhanced photocatalytic activity,” Mater. Res. Bull., vol. 107, pp. 194–203, Nov. 2018, doi: 10.1016/j.materresbull.2018.07.016.
CrossRef - A. D. Bachvarova-Nedelcheva, R. S. Iordanova, A. M. Stoyanova, R. D. Gegova, Y. B. Dimitriev, and A. R. Loukanov, “Photocatalytic properties of ZnO/TiO2 powders obtained via combustion gel method,” Cent. Eur. J. Chem., vol. 11, no. 3, pp. 364–370, Mar. 2013, doi: 10.2478/s11532-012-0167-2.
CrossRef - T. Aarthi and G. Madras, “Photocatalytic degradation of rhodamine dyes with nano-TiO2,” Ind. Eng. Chem. Res., vol. 46, no. 1, pp. 7–14, Jan. 2007, doi: 10.1021/ie060948n.
CrossRef - G. Zhou et al., “Surface plasmon resonance-enhanced solar-driven photocatalytic performance from Ag nanoparticles-decorated Ti3+ self-doped porous black TiO2 pillars,” J. Ind. Eng. Chem., vol. 64, pp. 188–193, Aug. 2018, doi: 10.1016/j.jiec.2018.03.015.
CrossRef - M. Cri?an et al., “Sol-gel iron-doped TiO2 nanopowders with photocatalytic activity,” Appl. Catal. A Gen., vol. 504, pp. 130–142, Sep. 2015, doi: 10.1016/j.apcata.2014.10.031.
CrossRef - M. I. Litter and J. A. Navío, “Photocatalytic properties of iron-doped titania semiconductors,” J. Photochem. Photobiol. A Chem., vol. 98, no. 3, pp. 171–181, Aug. 1996, doi: 10.1016/1010-6030(96)04343-2.
CrossRef - V. Caratto et al., “Different sol–gel preparations of iron-doped TiO2 nanoparticles: characterization, photocatalytic activity and cytotoxicity,” J. Sol-Gel Sci. Technol., vol. 80, no. 1, pp. 152–159, Oct. 2016, doi: 10.1007/s10971-016-4057-5.
CrossRef - S. Wang, J. S. Lian, W. T. Zheng, and Q. Jiang, “Photocatalytic property of Fe doped anatase and rutile TiO 2 nanocrystal particles prepared by sol-gel technique,” Appl. Surf. Sci., vol. 263, pp. 260–265, Dec. 2012, doi: 10.1016/j.apsusc.2012.09.040.
CrossRef - S. Sood, A. Umar, S. K. Mehta, and S. K. Kansal, “Highly effective Fe-doped TiO2 nanoparticles photocatalysts for visible-light driven photocatalytic degradation of toxic organic compounds,” J. Colloid Interface Sci., vol. 450, pp. 213–223, Jul. 2015, doi: 10.1016/j.jcis.2015.03.018.
CrossRef - H. Moradi, A. Eshaghi, S. R. Hosseini, and K. Ghani, “Fabrication of Fe-doped TiO2 nanoparticles and investigation of photocatalytic decolorization of reactive red 198 under visible light irradiation,” Ultrason. Sonochem., vol. 32, pp. 314–319, Sep. 2016, doi: 10.1016/j.ultsonch.2016.03.025.
CrossRef - A. M. Abdel-Wahab, A. S. Al-Shirbini, O. Mohamed, and O. Nasr, “Photocatalytic degradation of paracetamol over magnetic flower-like TiO2/Fe2O3 core-shell nanostructures,” J. Photochem. Photobiol. A Chem., vol. 347, pp. 186–198, Oct. 2017, doi: 10.1016/j.jphotochem.2017.07.030.
CrossRef - S. D. Delekar, H. M. Yadav, S. N. Achary, S. S. Meena, and S. H. Pawar, “Structural refinement and photocatalytic activity of Fe-doped anatase TiO 2 nanoparticles,” Appl. Surf. Sci., vol. 263, pp. 536–545, Dec. 2012, doi: 10.1016/j.apsusc.2012.09.102.
CrossRef - A. Khanna and V. Shetty K, “Solar photocatalysis for treatment of Acid Yellow-17 (AY-17) dye contaminated water using Ag@TiO2 core-shell structured nanoparticles,” Environ. Sci. Pollut. Res., vol. 20, no. 8, pp. 5692–5707, Aug. 2013, doi: 10.1007/s11356-013-1582-4.
CrossRef - M. Ye et al., “Magnetically Recoverable Core-Shell Nanocomposites with Enhanced Photocatalytic Activity,” Chem. - A Eur. J., vol. 16, no. 21, pp. 6243–6250, Apr. 2010, doi: 10.1002/chem.200903516.
CrossRef - M. Harikishore, M. Sandhyarani, K. Venkateswarlu, T. A. Nellaippan, and N. Rameshbabu, “Effect of Ag Doping on Antibacterial and Photocatalytic Activity of Nanocrystalline TiO 2,” Procedia Mater. Sci., vol. 6, pp. 557–566, Sep. 2014, doi: 10.1016/j.mspro.2014.07.071.
CrossRef - 66. A.S. Stasinakis, “(PDF) Use of Selected Advanced Oxidation Processes (AOPs) for Wastewater Treatment – a Mini Review,” Glob. Nest, vol. 10, no. 3, pp. 376–385, 2008.
CrossRef - A. Nezamzadeh-Ejhieh and S. Hushmandrad, “Solar photodecolorization of methylene blue by CuO/X zeolite as a heterogeneous catalyst,” Appl. Catal. A Gen., vol. 388, no. 1–2, pp. 149–159, Nov. 2010, doi: 10.1016/j.apcata.2010.08.042.
CrossRef - W. J. Sun, J. Li, G. P. Yao, M. Jiang, and F. X. Zhang, “Efficient photo-degradation of 4-nitrophenol by using new CuPp-TiO 2 photocatalyst under visible light irradiation,” Catal. Commun., vol. 16, no. 1, pp. 90–93, Nov. 2011, doi: 10.1016/j.catcom.2011.09.013.
CrossRef - A. Nezamzadeh-Ejhieh and A. Badri, “Surfactant modified ZSM-5 zeolite as an active component of membrane electrode towards thiocyanate,” Desalination, vol. 281, no. 1, pp. 248–256, Oct. 2011, doi: 10.1016/j.desal.2011.07.070.
CrossRef - A. Nezamzadeh-Ejhieh and N. Moazzeni, “Sunlight photodecolorization of a mixture of Methyl Orange and Bromocresol Green by CuS incorporated in a clinoptilolite zeolite as a heterogeneous catalyst,” J. Ind. Eng. Chem., vol. 19, no. 5, pp. 1433–1442, Sep. 2013, doi: 10.1016/j.jiec.2013.01.006.
CrossRef - A. Nezamzadeh-Ejhieh and M. Amiri, “CuO supported Clinoptilolite towards solar photocatalytic degradation of p-aminophenol,” Powder Technol., vol. 235, pp. 279–288, Feb. 2013, doi: 10.1016/j.powtec.2012.10.017.
CrossRef - M. Yasmina, K. Mourad, S. H. Mohammed, and C. Khaoula, “Treatment heterogeneous photocatalysis; Factors influencing the photocatalytic degradation by TiO2,” in Energy Procedia, 2014, vol. 50, pp. 559–566, doi: 10.1016/j.egypro.2014.06.068.
CrossRef - Y. Cao, H. Tan, T. Shi, T. Tang, and J. Li, “Preparation of Ag-doped TiO2 nanoparticles for photocatalytic degradation of acetamiprid in water,” J. Chem. Technol. Biotechnol., vol. 83, no. 4, pp. 546–552, Apr. 2008, doi: 10.1002/jctb.1831.
CrossRef - T. Kaur, A. Sraw, R. K. Wanchoo, and A. P. Toor, “Solar assisted degradation of carbendazim in water using clay beads immobilized with TiO2 & Fe doped TiO2,” Sol. Energy, vol. 162, pp. 45–56, Mar. 2018, doi: 10.1016/j.solener.2017.11.033.
CrossRef - T. V. R. Renugadevi1, S. P. , R. Narayanasamy, and P.Krishnamurthi, “Structural, optical properties and photocatalytic activity of Fe3C doped TiO2 thin films deposited by sol-gel spin coating - Google Search,” Rasayan J. Chem., vol. 9, no. 2, pp. 125–132, 2016.
- J. G. Mahy et al., “Towards a large scale aqueous sol-gel synthesis of doped TiO2: Study of various metallic dopings for the photocatalytic degradation of p-nitrophenol,” J. Photochem. Photobiol. A Chem., vol. 329, pp. 189–202, Oct. 2016, doi: 10.1016/j.jphotochem.2016.06.029.
CrossRef - M. B. Suwarnkar, R. S. Dhabbe, A. N. Kadam, and K. M. Garadkar, “Enhanced photocatalytic activity of Ag doped TiO2 nanoparticles synthesized by a microwave assisted method,” Ceram. Int., vol. 40, no. 4, pp. 5489–5496, May 2014, doi: 10.1016/j.ceramint.2013.10.137.
CrossRef - D. Sun, W. Sun, W. Yang, Q. Li, and J. K. Shang, “Efficient photocatalytic removal of aqueous NH4+-NH3 by palladium-modified nitrogen-doped titanium oxide nanoparticles under visible light illumination, even in weak alkaline solutions,” Chem. Eng. J., vol. 264, pp. 728–734, Mar. 2015, doi: 10.1016/j.cej.2014.12.012.
CrossRef - J. Lee, H. Park, and W. Choi, “Selective Photocatalytic Oxidation of NH 3 to N 2 on Platinized TiO 2 in Water,” Environ. Sci. Technol., vol. 36, no. 24, pp. 5462–5468, Dec. 2002, doi: 10.1021/es025930s.
CrossRef - X. C. & J. H. Xue Gong, Haifeng Wang, Chun Yang, Quan Li, “Treatment of ammonia nitrogen wastewater from coal gasification process with TiO2 photocatalysts doped with metal ions,” Futur. Cities Environ., vol. 1, no. 12, 2015.
- X. Gong, H. Wang, C. Yang, Q. Li, X. Chen, and J. Hu, “Photocatalytic degradation of high ammonia concentration wastewater by TiO2,” Futur. Cities Environ., vol. 1, no. 0, p. 12, 2017, doi: 10.1186/s40984-015-0012-9.
CrossRef - X. Luo et al., “Characterization of La/Fe/TiO2 and its photocatalytic performance in ammonia nitrogen wastewater,” Int. J. Environ. Res. Public Health, vol. 12, no. 11, pp. 14626–14639, Nov. 2015, doi: 10.3390/ijerph121114626.
CrossRef - A. Mukimin and H. Vistanty, “Hybrid advanced oxidation process (HAOP) as an effective pharmaceutical wastewater treatment,” E3S Web Conf., vol. 125, p. 03007, Oct. 2019, doi: 10.1051/E3SCONF/201912503007.
CrossRef - J. Madhavan, J. Theerthagiri, D. Balaji, S. Sunitha, M. Y. Choi, and M. Ashokkumar, “Hybrid Advanced Oxidation Processes Involving Ultrasound: An Overview,” Molecules, vol. 24, no. 18, Sep. 2019, doi: 10.3390/MOLECULES24183341.
CrossRef - S. Chakma, L. Das, and V. S. Moholkar, “Dye decolorization with hybrid advanced oxidation processes comprising sonolysis/Fenton-like/photo-ferrioxalate systems: A mechanistic investigation,” Sep. Purif. Technol., vol. 156, pp. 596–607, Dec. 2015, doi: 10.1016/J.SEPPUR.2015.10.055.
CrossRef - S. Patel, S. Mondal, S. K. Majumder, P. Das, and P. Ghosh, “Treatment of a Pharmaceutical Industrial Effluent by a Hybrid Process of Advanced Oxidation and Adsorption,” ACS Omega, vol. 5, no. 50, pp. 32305–32317, Dec. 2020, doi: 10.1021/ACSOMEGA.0C04139.
CrossRef - K. Roy and V. S. Moholkar, “p–nitrophenol degradation by hybrid advanced oxidation process of heterogeneous Fenton assisted hydrodynamic cavitation: Discernment of synergistic interactions and chemical mechanism,” Chemosphere, vol. 283, p. 131114, Nov. 2021, doi: 10.1016/J.CHEMOSPHERE.2021.131114.
CrossRef - K. Roy and V. S. Moholkar, “Mechanistic analysis of carbamazepine degradation in hybrid advanced oxidation process of hydrodynamic cavitation/UV/persulfate in the presence of ZnO/ZnFe2O4,” Sep. Purif. Technol., vol. 270, p. 118764, Sep. 2021, doi: 10.1016/J.SEPPUR.2021.118764.
CrossRef - M. Malakootian et al., “ZnO nanoparticles immobilized on the surface of stones to study the removal efficiency of 4-nitroaniline by the hybrid advanced oxidation process (UV/ZnO/O3),” J. Mol. Struct., vol. 1176, pp. 766–776, Jan. 2019, doi: 10.1016/J.MOLSTRUC.2018.09.033.
CrossRef - S. W. Lv, J. M. Liu, C. Y. Li, N. Zhao, Z. H. Wang, and S. Wang, “Two novel MOFs@COFs hybrid-based photocatalytic platforms coupling with sulfate radical-involved advanced oxidation processes for enhanced degradation of bisphenol A,” Chemosphere, vol. 243, p. 125378, Mar. 2020, doi: 10.1016/J.CHEMOSPHERE.2019.125378.
CrossRef - B. Skanes, J. Ho, K. Warriner, and R. S. Prosser, “Degradation of boscalid, pyraclostrobin, fenbuconazole, and glyphosate residues by an advanced oxidative process utilizing ultraviolet light and hydrogen peroxide,” J. Photochem. Photobiol. A Chem., vol. 418, p. 113382, Sep. 2021, doi: 10.1016/J.JPHOTOCHEM.2021.113382.
CrossRef - S. Dursun, S. N. Koyuncu, ?. C. Kaya, G. G. Kaya, V. Kalem, and H. Akyildiz, “Production of CuO–WO3 hybrids and their dye removal capacity/performance from wastewater by adsorption/photocatalysis,” J. Water Process Eng., vol. 36, p. 101390, Aug. 2020, doi: 10.1016/J.JWPE.2020.101390.
CrossRef - H. Chen, Y. J. Zhang, P. Y. He, C. J. Li, and H. Li, “Coupling of self-supporting geopolymer membrane with intercepted Cr(III) for dye wastewater treatment by hybrid photocatalysis and membrane separation,” Appl. Surf. Sci., vol. 515, p. 146024, Jun. 2020, doi: 10.1016/J.APSUSC.2020.146024.
CrossRef - H. B. Truong, B. T. Huy, S. K. Ray, Y. I. Lee, J. Cho, and J. Hur, “H2O2-assisted photocatalysis for removal of natural organic matter using nanosheet C3N4-WO3 composite under visible light and the hybrid system with ultrafiltration,” Chem. Eng. J., vol. 399, p. 125733, Nov. 2020, doi: 10.1016/J.CEJ.2020.125733.
CrossRef - T. Fazal et al., “Integrating adsorption and photocatalysis: A cost effective strategy for textile wastewater treatment using hybrid biochar-TiO2 composite,” J. Hazard. Mater., vol. 390, p. 121623, May 2020, doi: 10.1016/J.JHAZMAT.2019.121623.
CrossRef - S. Balu, Y. L. Chen, T. C. K. Yang, J. N. Chen, and S. W. Chen, “Effect of ultrasound-induced hydroxylation and exfoliation on P90–TiO2/g-C3N4 hybrids with enhanced optoelectronic properties for visible-light photocatalysis and electrochemical sensing,” Ceram. Int., vol. 46, no. 11, pp. 18002–18018, Aug. 2020, doi: 10.1016/J.CERAMINT.2020.04.115.
CrossRef






