Analysis of Soil and Water Quality in Selected Villages of Ranipet District, Tamil Nadu, India
Subramanian Arivoli1
 , Miriam Vassou2
, Miriam Vassou2
 , Samuel Tennyson3
*
, Samuel Tennyson3
*
 , Mohamed Meeran4
, Mohamed Meeran4
 , Athikesavan Ramanan1
, Athikesavan Ramanan1
 , Selvaraj Divya1
, Selvaraj Divya1
 and Pac Kamatchi5
and Pac Kamatchi5

1
Department of Zoology,
Thiruvalluvar University,
Vellore,
Tamil Nadu,
India
2
Department of Zoology,
Periyar EVR College,
Tiruchirappalli,
Tamil Nadu,
India
3
Department of Zoology,
Madras Christian College,
Chennai,
Tamil Nadu,
India
4
Department of Zoology,
Hajee Karutha Rowther Howdia College,
Uthamapalayam,
Tamil Nadu,
India
5
Department of Zoology,
Arignar Anna Govt. Arts College for Women,
Walajapet,
Tamil Nadu,
India
DOI: http://dx.doi.org/10.12944/CWE.16.2.14
Copy the following to cite this article:
Arivoli S, Vassou M, Tennyson S, Ramanan A, Divya S, Kamatchi P. Analysis of Soil and Water Quality in Selected Villages of Ranipet District, Tamil Nadu, India. Curr World Environ 2021;16(2). DOI:http://dx.doi.org/10.12944/CWE.16.2.14
Copy the following to cite this URL:
Arivoli S, Vassou M, Tennyson S, Ramanan A, Divya S, Kamatchi P. Analysis of Soil and Water Quality in Selected Villages of Ranipet District, Tamil Nadu, India. Curr World Environ 2021;16(2). Available From: https://bit.ly/3jrNyZR
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 12-03-2021 |
|---|---|
| Accepted: | 25-08-2021 |
| Reviewed by: | 
 Savalia S G Junagadh
Savalia S G Junagadh
|
| Second Review by: |

 Darwin H. Pangaribuan
Darwin H. Pangaribuan
|
| Final Approval by: | Dr. Umesh Chandra Kulshrestha |
Introduction
Soil and water quality are determined by estimating the concentration of their parameters since there exists solid relationships among various parameters and a joined impact of their inter-relatedness. Ecologically, Ranipet is the most strategic spot where many industries started mushrooming, and is strongly influenced by soil and water pollution of industrial origin. The Ranipet industrial region has been exceptionally tainted for long time due to unreasonable discarding of perilous wastes from industrial amenities and exhaust fumes. Consequently, due to the degree of geoaccumulation, contamination and assimilated pollution, excessive concentrations of pollutants found in the soils and water of the Ranipet industrial area, can be introduced into the food chain via soil and may be a genuine danger for human and animal well-being. High concentrations of metals in the Ranipet soils too may be blended due to industrial effluents. Researchers have provided details regarding the soil analysis of polluted sites of Ranipet.1-5 Studies on various sources of water report to the water quality of a village.6 Water quality too in Ranipet district is in a disturbing condition, and studies on ground water samples collected from wells was found to be fluctuating due to large number of tannery units and industrial activities.4 Nevertheless, the examination of the soil and water quality of Puliyanthangal and Kathiyavadi village respectively of Ranipet district has not been accounted for till date. Such assessment will feature the idea of soil and water for regular and other anthropogenic impacts, and along these lines, the soil and water quality was examined in the present study.
Materials and Methods
The study was done from January 2019 to December 2019. Sampling of soil and water was done in the early hours of morning, and transferred to the laboratory, with a minimum of three samples. The analysis of soil and water samples were done as per standard procedures.
Soil Analysis
The study area Puliyanthangal village (12.9180° N, 79.3336° E) is located 9Km away from Ranipet, Tamil Nadu, India and 1Km away from the tannery and industrial areas which incorporates leather and tanning industries, shoe factories, water effluent industries, ceramic industries, chemical factories, and drying electroplating industries. The portrayal of techniques of soil quality parameters to be analysed and compared with the standard permissible limits are presented in Table 1. Stratified random sampling was undertaken. The study area was divided into three zones viz., industrial area (zone 1), 10km from industrial area (zone 2), and agricultural land/fields (zone 3). In each zone, three sorts of soil samples, viz., top soil 0-10cm, subsoil 10-20 cm and inner soil 20-30cm were gathered for assessment. Soil parameters like soil texture, pH, electrical conductivity, organic carbon and macronutrients like nitrogen, phosphorous, potassium, and micronutrients like iron, manganese, zinc, and copper were examined. Sampling procedures for soil samples were strictly followed wherein, first a uniform slice of the soil was taken from the surface to the depth of the insertion, and secondly, a similar volume of soil was obtained in each sample. All soil sampling equipment’s were decontaminated prior to sample collection. Soil samples taken at a depth greater than three inches was collected with the aid of a hand auger. Care was taken that the soil sample was neither touched nor handled by bare hands. The collected samples were cleaned off for stones, plant residues and other unwanted materials if any, and then packed in plastic bags.
Water Analysis
The study area Kathiyavadi is a village (12.8850° N, 79.2747° E) in Arcot block, located 12Km away from Ranipet, Tamil Nadu, India and 3Km away from the tannery and industrial areas, encircled with tannery, ceramic, refractory, chromium and chemical industries. Kathiyavadi is encircled by Walajapet block towards north, Timiri block towards south, Sholinghur block towards north, Arani block towards south. The depiction of techniques of water quality parameters to be analysed and compared with the standard permissible limits of water quality are presented in Table 2. Three different sources of water, viz., bore well, well and pond samples were analysed for their physicochemical (appearance, colour, odour, turbidity, total dissolved solids, electrical conductivity, pH, total alkalinity, total hardness), nutrients (calcium, magnesium, free ammonia, nitrite, nitrate, chloride, fluoride, sulphate, phosphate), and metal (iron and manganese) properties. High-density polyethylene bottles were used to collect the water samples (1L) after rinsing with the sample twice/thrice.
 |
Figure 1: Map of the Study Area. Click here to view Figure |
Statistical Analysis
Correlation matrix analysis was performed utilizing SPSS software programme to determine the relationship between parameters responsible for influencing the soil and water quality of the study area using Pearson’s linear correlation with P<0.05 significant threshold.
Table 1: Procedure and Permissible Limits for each Soil Parameter set by Standards.
|
Parameters |
Unit |
Method |
BIS |
IASS |
|
Physicochemical |
||||
|
Texture |
- |
Hydrometer |
- |
- |
|
pH |
- |
Systronic digital pH meter |
6.8-8.0 |
4.0-8.5 |
|
Electrical conductivity |
dS/m |
Conductivity meter |
0.07-0.6 |
4.0 |
|
Organic carbon |
% |
Potentiometric titration |
0.5-7.5 |
0.5-5.5 |
|
Macronutrients |
||||
|
Nitrogen |
mg/Kg |
Kelplus Distyl - EMS |
<50 |
280-560 |
|
Phosphorous |
Colorimeter |
<2.5 |
>7 |
|
|
Potassium |
Flame photometer |
<60 |
>80 |
|
|
Micronutrients |
||||
|
Iron |
mg/Kg |
Atomic absorption spectrophotometer |
2.9-6.7 |
4.1 |
|
Manganese |
3.8-6.9 |
5.0 |
||
|
Zinc |
0.3-1.4 |
2.5 |
||
|
Copper |
0.3-1.5 |
2.0 |
||
BIS: Bureau of Indian Standards; IASS: International Agricultural Soil Standards
Table 2: Procedure and Permissible Limits for each Water Parameter Set by Standards.
|
Parameters |
Unit |
Method |
BIS |
CPCB |
ICMR |
ISI |
USEPA |
WHO |
|
Physicochemical |
||||||||
|
Colour/Appearance |
Hazen |
Visual comparison |
- |
- |
- |
5 |
- |
- |
|
Odour |
- |
Physiological sense |
Acceptable |
Acceptable |
Acceptable |
Acceptable |
- |
Acceptable |
|
Temperature |
°C |
Mercury-in-glass thermometer |
- |
- |
- |
- |
- |
- |
|
Electrical conductivity |
?S/cm |
Conductivity meter |
- |
2000 |
- |
- |
- |
1500 |
|
Turbidity |
NTU |
Nephelometric turbidity meter |
10 |
10 |
25 |
10 |
- |
<5 |
|
Total dissolved solids |
mg/L |
Ion selective |
2000 |
- |
500 |
1500 |
- |
1000 |
|
pH |
- |
Systronic digital pH meter |
6.5-8.5 |
6.5-8.5 |
6.5-8.5 |
6.5-9.2 |
6.5-8.5 |
6.5-8.5 |
|
Total alkalinity |
ppt |
Acid titration |
200 |
600 |
- |
200 |
200 |
120 |
|
Total hardness |
EDTA titration |
300 |
600 |
600 |
300 |
- |
500 |
|
|
Nutrients |
||||||||
|
Calcium |
mg/L |
Flame photometer |
200 |
200 |
200 |
200 |
- |
200 |
|
Magnesium |
Complexometric EDTA titration |
100 |
- |
- |
100 |
- |
150 |
|
|
Free ammonia |
Complexometric EDTA titration |
- |
- |
- |
1.5 |
- |
0.5 |
|
|
Nitrate |
UV visible spectrophotometer |
45 |
100 |
100 |
45 |
50 |
45 |
|
|
Nitrite |
- |
- |
- |
45 |
0.5 |
3 |
||
|
Chloride |
Argentometric titration |
1000 |
1000 |
1000 |
1000 |
250 |
600 |
|
|
Fluoride |
SPADNS spectrophotometer |
1.5 |
1.5 |
1.5 |
0.6-1.2 |
4 |
1.5 |
|
|
Sulphate |
Nephelometer and Turbidimeter |
400 |
400 |
400 |
400 |
250 |
250 |
|
|
Phosphate |
Stannous chloride |
- |
- |
- |
- |
- |
0.1 |
|
|
Metals |
||||||||
|
Iron |
mg/L |
Atomic absorption spectrophotometer |
1 |
1 |
1 |
0.3 |
- |
0.1 |
|
Manganese |
30 |
100 |
- |
30 |
- |
50 |
||
Results
The texture of soil was sandy loam in all the three zones analysed, and the results of soil parameters, viz., pH, electrical conductivity, organic carbon, and macronutrients like nitrogen, phosphorous, potassium, and micronutrients like iron, manganese, zinc, and copper tested in the top, sub and inner soil of zone 1 reported values of 8.6, 8.1 and 7.1; 0.4, 0.2 and 0.2; 0.03, 0.03 and 0.2; 15.0, 10.0 and 10.0; 4.0, 1.5 and 8.0; 102.0, 65.0 and 98.0; 4.3, 3.3 and 3.3; 2.3, 2.0 and 2.0; 0.5, 0.4 and 0.4; and 0.8, 0.8 and 0.6, respectively. For zone 2, the respective values were 7.1, 6.3 and 6.9; 0.2, 0.4 and 0.2; 0.4, 0.5 and 0.6; 72.0, 96.0 and 120.0; 5.5, 4.0 and 4.0; 147.0, 85.0 and 102.0; 3.9, 3.7 and 3.4; 2.5, 2.2 and 2.1; 0.2, 0.2 and 0.1; and 0.9, 0.7 and 0.6. In the case of zone 3, the values were 6.9, 6.6 and 6.8; 0.1, 0.2 and 0.1; 0.6, 0.5 and 0.4; 150.0, 150.0 and 113.0; 3.0, 5.4 and 4.0; 230.0, 275.0 and 115.0; 3.9, 3.2 and 3.1; 2.1, 1.6 and 1.4; 0.5, 0.3 and 0.2; and 0.7, 0.5 and 0.4, respectively (Figure 2). Table 3 displays the correlation matrix of the soil parameters at P<0.05 level.
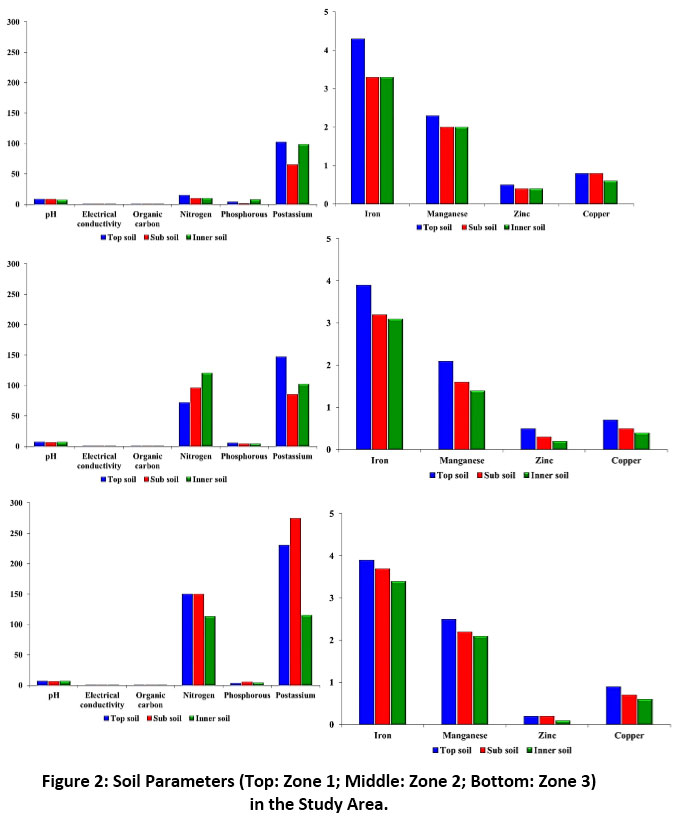 |
Figure 2: Soil Parameters (Top: Zone 1; Middle: Zone 2; Bottom: Zone 3) in the Study Area. Click here to view Figure |
Table 3: Correlation Matrix of Soil Parameters in the Study Area.
|
|
pH |
EC |
OC |
NI |
PH |
PO |
IR |
MA |
ZI |
CO |
|
Zone 1 |
||||||||||
|
pH |
1 |
|
||||||||
|
EC |
0.75 |
1 |
|
|||||||
|
OC |
-0.94 |
-0.5 |
1 |
|
|
|
|
|
|
|
|
NI |
0.75 |
1 |
-0.5 |
1 |
||||||
|
PH |
-0.74 |
-0.13 |
0.92* |
-0.13 |
1 |
|||||
|
PO |
-0.09 |
0.58 |
0.41 |
0.58 |
0.72 |
1 |
||||
|
IR |
0.75 |
1 |
-0.5 |
1 |
-0.13 |
0.58 |
1 |
|||
|
MA |
0.75 |
1 |
-0.5 |
1 |
-0.13 |
0.58 |
1 |
1 |
||
|
ZI |
0.75 |
1 |
-0.5 |
1 |
-0.13 |
0.58 |
1 |
1 |
1 |
|
|
CO |
0.94* |
0.5 |
-1 |
0.5 |
-0.92 |
-0.41 |
0.5 |
0.5 |
0.5 |
1 |
|
Zone 2 |
||||||||||
|
pH |
1 |
|
|
|
|
|
|
|
|
|
|
EC |
-0.97 |
1 |
|
|
|
|
|
|
|
|
|
OC |
-0.24 |
0 |
1 |
|
|
|
|
|
|
|
|
NI |
-0.24 |
0 |
1 |
1 |
|
|
|
|
|
|
|
PH |
0.69 |
-0.5 |
-0.86 |
-0.86 |
1 |
|
|
|
|
|
|
PO |
0.86* |
-0.71 |
-0.70 |
-0.70 |
0.96* |
1 |
|
|
|
|
|
IR |
0.12 |
0.11 |
-0.99 |
-0.99 |
0.80* |
0.61 |
1 |
|
|
|
|
MA |
0.5 |
-0.27 |
-0.96 |
-0.96 |
0.97* |
0.87 |
0.92* |
1 |
|
|
|
ZI |
-0.27 |
0.5 |
-0.86 |
-0.86 |
0.5 |
0.25 |
0.91* |
0.69 |
1 |
|
|
CO |
0.41 |
-0.18 |
-0.98 |
-0.98 |
0.94* |
0.82 |
0.95* |
0.99* |
0.75 |
1 |
|
Zone 3 |
||||||||||
|
pH |
1 |
|
|
|
|
|
|
|
|
|
|
EC |
-0.94 |
1 |
|
|
|
|
|
|
|
|
|
OC |
0.32 |
0 |
1 |
|
|
|
|
|
|
|
|
NI |
-0.18 |
0.5 |
0.86* |
1 |
|
|
|
|
|
|
|
PH |
-0.99 |
0.91* |
-0.41 |
0.09 |
1 |
|
|
|
|
|
|
PO |
-0.45 |
0.71 |
0.69 |
0.96* |
0.36 |
1 |
|
|
|
|
|
IR |
0.67 |
-0.39 |
0.91* |
0.59 |
-0.74 |
0.35 |
1 |
|
|
|
|
MA |
0.54 |
-0.24 |
0.97* |
0.72 |
-0.62 |
0.50 |
0.98* |
1 |
|
|
|
ZI |
0.5 |
-0.18 |
0.98* |
0.75 |
-0.57 |
0.54 |
0.97* |
0.99* |
1 |
|
|
CO |
0.5 |
-0.18 |
0.98* |
0.75 |
-0.57 |
0.54 |
0.97* |
0.99* |
1 |
1 |
*Correlation significant @ P<0.05
pH: Hydrogen ion concentration; EC: Electrical conductivity; OC: Organic carbon;
NI: Nitrogen; PH: Phosphorous; PO: Potassium; IR: Iron; MA: Manganese; ZI: Zinc; CO: Copper
The results of the physicochemical and nutrient parameters of bore well, well and pond water samples in the study area showed marked differences amongst them. The bore well, and well water samples were clear and colourless without odour, whereas the pond water was slightly yellowish in appearance and colour, and without odour in all the three zones. The physicochemical parameters viz., water temperature, electrical conductivity, turbidity, total dissolved solids, pH, total alkalinity and total hardness for bore well, well and pond water were 27.9, 22.8 and 33.0°C; 975, 1532 and 737?S/cm; 0, 1 and 11NTU; 683, 1072 and 516mg/L; 7.4, 7.8 and 7.3; 276, 344 and 248mg/L; and 190, 732 and 272mg/L respectively (Figure 3). Calcium, magnesium, free ammonia, nitrate, nitrite, chloride, fluoride, sulphate and phosphate represented the nutrient parameters and their respective values (mg/L) were 41, 174 and 58; 21, 71 and 30; 0, 0 and 1.1; 24, 29 and 22; 0, 0 and 0.8; 100, 184 and 60; 0.4, 0.4 and 0.4; 77, 120 and 49; 0, 0 and 0.8. The values of metal parameters reported nil except for iron (0.2mg/L) in pond water (Figure 4). Table 4 displays the correlation matrix of the water parameters at P<0.05 level.
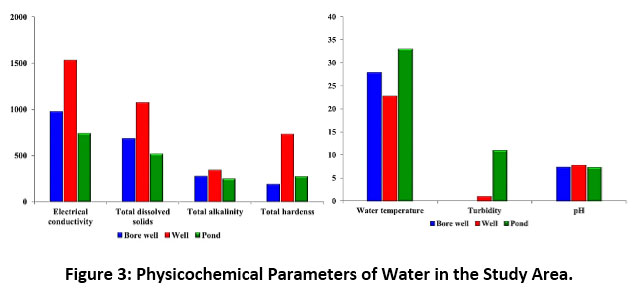 |
Figure 3: Physicochemical Parameters of Water in the Study Area. Click here to view Figure |
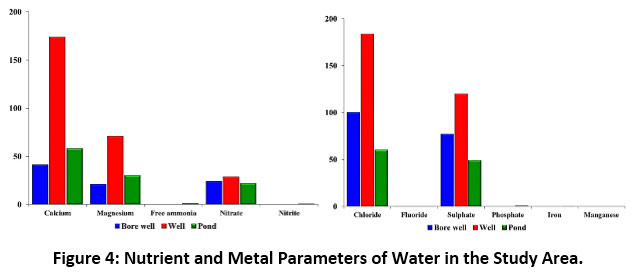 |
Figure 4: Nutrient and Metal Parameters of Water in the Study Area. Click here to view Figure |
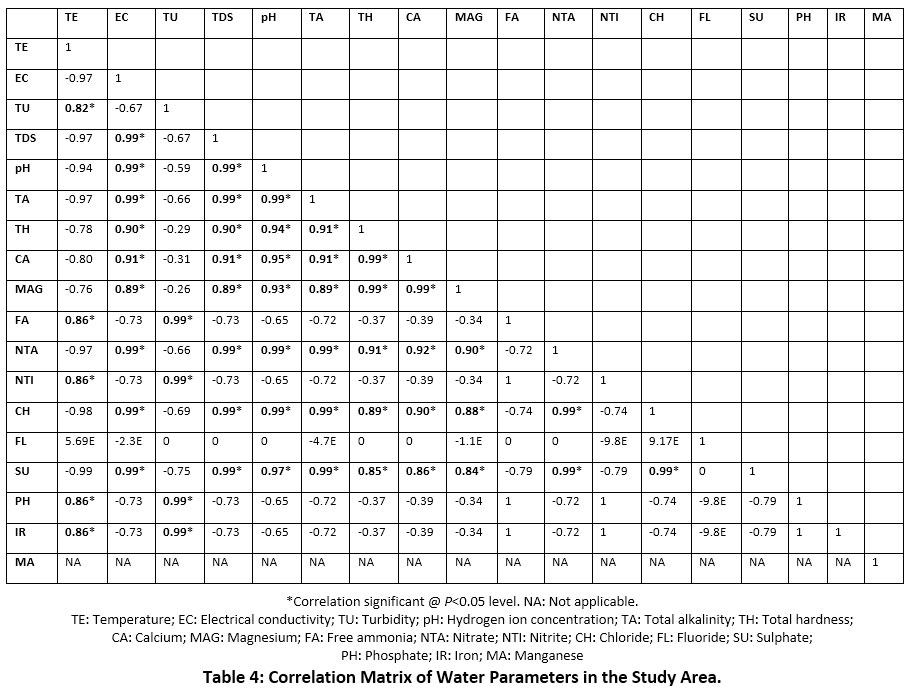 |
Table 4: Correlation Matrix of Water Parameters in the Study Area. Click here to view Figure |
Discussion
Soil is a significant component of human biosphere, and is the skin of the earth on which plants develop, and any unsafe change to this environment genuinely influences the quality of life.2 They help in ecosystem services, like, water filter, carbon sink, atmospheric gases regulation, and medium for plant growth, which helps to sustain life.7 The nature of soil rely upon different substance components and their fixation is developed from the topographical information of a specific village/area/district. By and large, the nature of soil relies upon the collaboration between soil and water, soil-gas association, rocks with which it comes into contact in the unsaturated zone, and reactions that occur inside. Soil quality in an area is largely to a great extent directed by disintegration and precipitation of minerals, water speed, nature of revive water, and collaboration with different kinds of water spring, and anthropogenic exercises.8
Physical and chemical assessment of soil relate the available soil macro and micronutrients with the soil properties.9 Soil texture is the fundamental pointer of its physical and chemical properties, and in the present study the texture was sandy loam in all the zones. Soils with heavy texture or soils with marked textural changes in profile are more susceptible to salinization and have drainage and reclamation problems.10 pH is one of the central factor of the soil with which its condition is classified as suitable or non-suitable (<8.5) for crop cultivation.11 In the present study, the values were 7.9, 6.8 and 6.8 for the three respective zones, with zone one value nearing non-suitable limit attributable to the presence of industrial areas, and its surrounding might be owed to the high organic matter which tends to buffer the soil by precluding extreme pH alterations owing to the release of exchangeable cations through mineralization of organic matter. The increased pH in the industrial area is a positive efficiency marker in tropical acidic soils where low pH confines nutrient element uptake,2 and its alkaline content might be due to higher degree of base saturation.12,13 Soil electrical conductivity is a significant pointer of soil wellbeing, wherein acidity and salinity of soils are measured, in addition to plant development with regard to nitrogen content which is its key aspect.14 It is a backhanded estimation that corresponds very well with several physical and chemical properties of soils. At the point when electrical conductivity is kept up at the legitimate level, plants supplements are at greatest accessibility, toxic elements at abridged accessibility, and useful soil flora/fauna are most energetic,15 while more significant level of electrical conductivity influences plant supplement accessibility, crop yields and action of soil microorganisms. The values reported at all three zones was within the permissible limits in the present study. Organic carbon plays an important role in soil biology by releasing nutrients to plants, providing buffering to soil and stabilizing the soil structure. Organic carbon status of the soil in three zones ranged between 0.1 and 0.3% which indicated low fertility due to high temperature, leading to higher rate of organic matter decomposition, and also due to little or no organic matter additions.16 High values may be due to decay of plants recedes in soil and can cause damage to sensitive plants like onions. However, deprived flora, and high proportion of decayed organic matter under hyper-thermic temperature indicates high oxidizing environment at very low content (<0.5%).15
Macronutrients have a major role in the soil. Nitrogen is a constituent of chlorophyll, which surges vegetative growth of protein content, and cation exchange capacity of plant roots. Deficiency of nitrogen will influence the metabolic activity of the plants bringing about hindered development, shorter internodes, light green and pale yellow leaves, and shedding of leaves and fruits.7,17 Excess of nitrogen is also a problem to the plant which will be reflected by the deferred development and defenselessness to insects and diseases. In the present study, the value of nitrogen was very low in zone 1 when compared to zones 2 and 3 which were several times higher. Phosphorous, vital for seed germination and fundamental for blossoming and formation of fruits, offer energy by photosynthesis to drive metabolism, and helps in energy stockpiling and transport in plants. An insufficiency of this nutrient can prompt weakened vegetative growth, disturbance in the nitrogen metabolism of plants, weak root systems, formation of purple stems and leaves, resulting in poor yield of fruits and seeds.7,9,15 In the present study, the value of phosphorous in all zones was within the permissible limits. Potassium considered as the quality nutrient is required in large amounts as it controls the opening and closing of stomata, uptake of carbon dioxide, and for proper growth and reproduction of plants. Potassium additionally triggers development related protein initiate on, and is fundamental for the creation of adenosine triphosphate, besides osmoregulation.7 Plants lacking potassium cannot exploit resourcefully nitrogen and water, and are vulnerable to chlorosis, defoliation, deliberate/diminutive development, feeble unhealthy roots, irregular maturing of fruits, and poor resistance to temperature changes, drought, and pests.15 In the present study, their values were above the limits in all zones.
Micronutrients needed in very small amounts plays an imperative part in upholding the health of soil and crop productivity. However, their inadequacies have become significant imperatives to efficiency, stability and sustainability of soils.18 Iron is a fundamental mineral that is needed for plant growth,9 as it helps in chlorophyll formation, and protein synthesis, acts as a module of numerous enzymes related with transfer of energy, reduction of nitrogen, and fixation of lignin. At low concentration, it causes yellowing of new leaves and green vein occurs, and in excess causes tiny brown spots on lower side of leaves. Manganese is crucial for nitrogen metabolism and is liable for the production of molecular oxygen in plants during photosynthesis. It acts as a catalyst in oxidation-reduction reaction, activator of many enzymes and helps in chlorophyll synthesis. Brown spots on the veins of the leaf blade and leaf sheath, and stunted growth are indicative of its excess content which might be due to decomposition of organic matter which releases micronutrients and also reduces the pH of the soil around the plant roots which helps in increasing the solubility of cationic micronutrients.19 Its deficiency leads to interveinal chlorosis which is a characteristic symptom, accompanied by infertility. Zinc is naturally present in the soil, however, its concentrations increase uncharacteristically, owing to industrial/anthropogenic activities like mining, coal, waste combustion, and steel processing. It helps in the formation of growth hormones, enhances heat and frost resistance and acts as catalyst in chlorophyll formation. Granular texture, raised pH, calcareousness, waning organic carbon, and leaching frequently highlight zinc deficiency which displays stunted growth, khaira disease and delayed maturity, and on the other hand, excessive uptake of zinc causes stunting, curling, rolling of young leaves, death of leaf tops and chlorosis in plants.2 Copper plays a vital role in root metabolism and is essential for metabolism of nitrogen, cell wall strength and prevention of wilting through lignin synthesis, besides formation of proteins and amino acids, and oxidation-reduction reaction. Its deficiency, causes the tip of the leaves to turn to white, and its toxicity disturbs mitosis, inhibits root elongation, and damages root cell membrane.20 Nonetheless, all the values of these micronutrients in all the three zones were within the standard limits except for manganese which was lower.
Water quality in an area is generally by and large directed by disintegration and precipitation of minerals, water speed, nature of revive water, and collaboration with different kinds of water spring, and anthropogenic activities.21 One of the most important physicochemical feature of any water is temperature as they are affected by rainfall and accessibility of light. Temperature influences the chemical, biochemical and biological characteristics of the aquatic system and indirectly regulates the colouration in aquatic organisms, especially fishes. High temperatures profoundly affect the physicochemical properties, and the biotic spectrum present within the water. Low temperature brings about the darkening effect, while its rise results in the concentration of pigments with consequent lightning of the colour.22 In the present study, temperature of effluent, corporation and bore well water was 27.9, 22.8 and 33.0°C respectively. Naturally, water remains at low temperature and evaporates to high temperatures. Seasonal and diurnal variations in temperature of natural water rarely cause any problems. High temperatures in natural waters are recorded when heated waters or effluents are discharged from industries or power plants which was observed in the present study.
Water turns into a conveyor of electric current when substances are dissolved in it and the conductivity is proportionate to the measure of disintegrated substances. Electrical conductivity is an extent of water capacity to pass on electric energy and relies upon the presence of deteriorated particles in water.23 This parameter is valuable to assess the purity of water and is constrained by topography of the territory where the water body is located.24 Conductivity displays critical relationship with different parameters, viz., temperature, pH, alkalinity, total hardness, calcium, total suspended solids, total dissolved solids, chemical oxygen demand, and concentration of chloride and iron in water. Moreover, Sooraj 25 stated that more the concentration of dissolved solids, greater the conductivity of water would be. Nonetheless, the values for all water samples in the present study did not cross the standard limits.
Pure water has a slightly blue colour that becomes a deeper blue as the thickness of the observed sample increases due to turbidity.22 Assessment of turbidity is a critical preliminary of water quality instigated by suspended matter, like clay, organic and inorganic matter finely divided, coloured soluble organic compounds, and other microscopic organisms.26 The suspended solids and dissolved matter impart turbidity to natural waters, and hence the penetration of light to lower layers of water gets restricted which harm aquatic life and surface water quality. During monsoon, turbidity is increased due to disintegration of substantial soil and suspended solids from sewage,27 which can impose threat to aquatic life. Therefore, in turbid waters, plant life in subsurface layers of water is reduced in addition to oxygen production in water. Turbidity also reduces the general usefulness of water and its high values reduce the filter runs which forms the basis for pathogenic microorganisms to be more perilous to the human life, and the values of the present study was within the permissible limits set by standards.
Total dissolved solids is a critical factor for assessing the proportion of strong solid materials deteriorated in the water which gives an odd taste to water and reduces its potability as well. It confers hardness to the water concerned which meddles with froth forming capacity of the water when utilized for cleaning purposes. High values of total dissolved solids may be due to water pollution when waste waters from both residential and industrial areas are discharged into it.28 WHO stipulated the limits to be 1000mg/L and the present study indicated that the total dissolved solids did not exceed the limitations of standards, except for slight increase in well water.
pH is a quintessential biogeochemical parameter, which plays a significant part in natural processes and is of widespread importance in ecosystem. The pH of water is critical to survival of most aquatic plants and animals. pH is reflected as a noteworthy characteristic factor and conveys information on various kinds of geochemical balance,29 and is the and is the significant concluding segment to water destructiveness.22 Many species cannot endure if pH drops below five or rises above nine, as they can alter the chemistry of water. ICMR and WHO has stipulated a pH range 6.5-8.5 as the recommended limit. In the present investigation, the pH values were 7.2 for effluent and bore well water and 7.9 for corporation water. pH in all samples was neutral to alkaline and variations in pH are moderately little in the present study, which denotes soils with weak basic salts.30
Alkalinity alludes to the water capacity to neutralise the acid,25 and is important as knowledge of this parameter provides useful information about bicarbonate, carbonate and hydroxide content of natural waters. Alkalinity in water comes from calcium carbonate, being leached from soil,26 and alkaline waters are usually more productive as they are able to carry a greater load of dissolved solids. The alkalinity for all water samples in the present study was above the permissible limits set by standards. Alkalinity in large measure, imparts unpleasant taste to water and eye irritation in human.31
Total hardness is the estimation of mineral content in water and addresses the total content of chlorides, sulphates, bicarbonates and carbonates.25 Hard water have an undesirable taste and little utility,32 and the estimates of the present study were within the standard limits except for well water indicative of hard water.
Distribution of nutrients is due to seasonal, tidal circumstances and freshwater movement from terrestrial sources. Excessive amount of nutrients might be owed to substantial entry of freshwater derived from land seepage, electroplating, tanning, dyeing and textile manufacturing industries. Calcium and magnesium decide the hardness of water and are considered as micronutrients that impact the growth and distribution of plant and animals,33 and their values recorded in the present study were in concurrence with the standard limits. Ions of calcium and magnesium make up the total hardness and henceforth are unified. Calcium has been a basic parameter for detecting contamination of water by sewage plant prior to bacteriological technique. Calcium in water can be produced from silicate minerals hydrolysis, contributing to hardness, thus influencing the organoleptic water quality. Whereas, untreated household sewage and industrial wastes are viewed as significant wellspring of magnesium which is at risk for hardness of water.34
The levels of free ammonia in the present study were nil except for pond water (1.1mg/L). Free ammonia in soil and aquatic environs constitute the end degraded product of organic and inorganic matter since it is the most reduced form of nitrogen. Its presence in percolation indicates anthropogenic contamination, in addition to bacterial activity of the soil, agriculture and industrial wastes.35 Nitrogen exists in the soil in reduced, oxidized or organic forms,36 and agricultural activities increase nitrate concentration in ground and surface water.37 Nitrate essential for plant growth is an oxidized form of nitrogen present in the soil. Increased nitrate amounts in water leads to decreased oxygen level, disturbing aquatic life, plants and algae,38 and further can change normal haemoglobin to methaemoglobin.39 However, the values of nitrates in the present study fell within the limits for all water samples. Nitrites originate from degradation of organic matter, reduction of nitrates and the oxidation of ammonia, and in excess poses health hazards due to their toxic oxidizing power. Nevertheless, they were nil except for pond water which was within the standard limit.
Chloride is present in all types of water naturally owing to activities from agriculture, industries, and rocks rich in chloride. Chloride focus relies upon the attributes of pollution load and fills in as a marker of contamination by sewage, and fertilizers return from agricultural fields. High chloride fixation are markers of large amount of organic matter in water, and lower chloride content in unpolluted waters. Despite the fact that chloride is less unsafe, it still gives unpleasant taste to the water, and damages the floral vegetation.25 The chloride values for all water samples of the present study was well below the standard limits.
Fluoride values reported 0.4mg/L for all samples in the present study which was within the permissible limits. Fluoride ions have high importance in water quality monitoring. High fluoride content in water leads to commencement of tooth decay, upsurges tooth enamel resistance against acids, skeletal damage and bone disease and when low it causes discolouration of teeth.40
Sulphate occurs in trifling quantities in the form of sulphate fertilizers owing to industrial/anthropogenic activities. Sulphate is obtained from the disintegration of salts of sulphuric acid which are found plentifully in all water bodies. High concentration of sulphate may be due to oxidation of pyrite and mine drainage.41 The respective values for all water samples in the present study were well below the permissible limits.
All phosphorus containing compounds yield inorganic phosphate after decomposition, and majority of phosphates come from metabolic breakdown of proteins and phosphates in urine. The major input of phosphates into an aquatic ecosystem comes from the domestic sewage, detergents, residual fertilizers, agricultural products, and industrial wastes.42 Pollution due to industrial and sewage waste contains phosphates aids in growth of microorganisms. Phosphate enters into surface waters with storm water runoff from agricultural fertilizers or residential cultivated land. High phosphate level causes damage of muscles, failure of kidney, and breathing problem,43 besides eutrophication and exhaustion of dissolved oxygen.38 Nonetheless, they were nil except for pond water which was above the standard limits indicative of industrial effluents.
Entry of iron into the water body may be through the tinkering and electroplating shops, paint factory, electrical engineering works contributing to the increase in the heavy metal content. Iron is one of the most fundamental component of blood in human and other living organism and is essential for nutrition and metabolism, nevertheless in excess leads to hemochromatosis in tissues.22,39 Iron content was recorded nil apart from pond water which lie in par with the permissible limits. Manganese is present as manganous ion in water and its high concentration may cause some adverse health effects,44 but in the present study their values were nill.
Conclusion
The nature of soil and water tested from Puliyanthangal and Kathiyavadi villages of Ranipet district respectively has been conceivable to comprehend the nature of soil and water in the examination region and to assess its appropriateness. The study surmised that the water parameters in the study area were fluctuating, within, in par and above the permissible limits. Further, the purpose behind waning nature of soil and water might be due to location of industrial units. Future studies on soil and water analysis of other villages in Ranipet district would enhance the understanding of soil and water quality, and on land evaluation too.
Acknowledgments
The authors are thankful to the authorities of Thiruvalluvar University, Vellore, Tamil Nadu, India for extending research facilities to analyse the samples.
Funding Sources
The authors received no financial support for this research.
Conflict of Interest
The authors declare no conflict of interest.
References
- Andiyappan K, Alagarsamy V.A.V, Abubacker T.A. Contemporary status of heavy metal contamination in soils affected by tannery activities, Ranipet, south India. Oriental Journal of Chemistry 2017; 33(6): 3092-3100.
CrossRef - Sharpudin J, Pratheesh, B, Balakumar K, Tamilthendral V. Spatial Variation and an Investigation on soil quality in the vicinity of industrial area in Ranipet, Vellore. International Journal of Advanced Research Trends in Engineering and Technology 2018; 5(5): 50-60.
- Thangam T.E.D, Kumar V.N, Vasline Y.A. Remediation of chromium contamination in and around Tamil Nadu Chromate Chemicals Limited in SIPCOT industrial estate, Ranipet, Vellore district, Tamil Nadu, India. International Journal of Applied Engineering Research 2018; 13(7): 4878-4883.
- Arivoli S, Samuel T, Manimegalai G, Vigneshkumar E, Meeran M, Marin G, Vassou M, Divya S, Kamatchi P.A.C. Assessment of soil and water quality and its possible impact on the flora and fauna in Jayarampettai village of Ranipet district, Tamil Nadu, India. Uttar Pradesh Journal of Zoology 2020; 41(23): 62-71.
- Kistan A, Kanchana V. Cr and Pb contamination in agricultural soil in two different seasons and three depth of the soil layer samples nearby tannery waste disposal zones at Ranipet, Vellore district in the southern India. International Journal of Pharmaceutical Sciences and Research 2020; 11(7): 3469-3473.
- Sivachandrabose K, Dhanalakshmi S. Studies on the physico-chemical arameters of bore well, well, pond and tap waters in Kambarajapuram village of Vellore district, Tamil Nadu (India). International Journal of Zoology and Animal Biology 2020; 3(6): 000254.
- Subha T.J, Rose G.L. Physico-chemical parameters and fertility status in selected soils of Agastheeswaram, Kalkulam and Vilavancode taluk’s of Kanyakumari district, Tamil Nadu, India: A study. Indian Journal of Advances in Chemical Science 2016; 4(4): 386-393.
- Schoonover J.E, Crim J.F. An introduction to soil concepts and the role of soils in watershed management. Journal of Contemporary Water Research & Education 2015; 154(1): 21-47.
CrossRef - Sheeja J.L.P. Assessment of macro and micronutrients in soils from Mannargudi area, Thiruvarur district, Tamil Nadu, India. Research Journal of Chemical and Environmental Sciences 2015; 3(6): 32-37.
- Vijayalakshmi M, Maniyosai R. An analysis on the soil characteristics of Perambalur district in Tamil Nadu. Journal of Information and Computational Science 2020; 10(3): 1274-1287.
- Hemageetha N, Nasira G.M. Analysis of soil condition based on pH value using classification techniques. IOSR Journal of Computer Engineering 2016; 18(6): 50-54.
- Sharma P.K, Sood A, Setia R.K, Tur N.S, Mehra D, Singh H. Mapping of macronutrients in soils of Amritsar district (Punjab) – A GIS approach. Journal of the Indian Society of Soil Science 2008; 56: 34-41.
- Waghmare M.S, Indulkar B.S, Mali C.V, Takankhar V.G, Bavalgave V.G. Chemical properties and micronutrients status of some soils of Ausa tehsil of Latur, Maharashtra. Asian Journal of Soil Science 2008; 3: 236-241.
- Borbe A.M.A.A, Doran J.W, Drijber R.A, Dobermann A. Soil electrical conductivity and water content affect nitrous oxide and carbon dioxide emissions in intensively managed soils. Journal of Environmental Quality 2006; 35: 1999-2010.
CrossRef - Raman N, Sathiyanarayanan D. Physico-chemical characteristics of soil influence of cation exchange capacity of soil in and around Chennai. Rasayan Journal of Chemistry 2009; 2(4): 875-885.
- Rego T.J, Rao V.N, Seeling B, Pardhasaradhi G, Rao K.J.V.D.K. Nutrient balances a guide to improving sorghum and groundnut based dry land cropping systems in semiarid tropical India. Field Crops Research 2003; 81: 53-68.
CrossRef - Shivanna A.M, Nagendrappa G. Chemical analysis of soil samples to evaluate the soil fertility status of selected command areas of three tanks in Tiptur taluk of Karnataka, India. IOSR Journal of Applied Chemistry 2014; 11(1): 1-5.
CrossRef - Vijayakumar R, Arokiaraj A, Prasath M.D.P. Micronutrients status and their relation to soil characteristics of south-east coastal soils of India. International Journal of Research in Chemistry and Environment 2011; 1(1): 147-150.
- Sharma J.C, Chaudhary K. Vertical distribution of micronutrient cations in relation to soil characteristics in lower Shiwaliks of Solan district in north-west Himalayas. Journal of the Indian Society of Soil Science 2007; 55: 40-44.
- Onder S, Dursun S, Gezgin S, Demirbas A. Determination of heavy metal pollution in grass and soil of city centre green areas (Konya, Turkey). Polish Journal of Environmental Studies 2007; 16(1): 145-154.
- Andrade E, Palacio H.A.Q, Souza I.H, Leao R.A, Guerreio M.J. Land use effects in groundwater composition of an alluvial aquifer by multivariate techniques. Environmental Research 2008; 106: 170-177.
CrossRef - Kumar A, Garg V. Heavy metal and physico-chemical characteristics of river Ganga from Rishikesh to Brijghat, India. Journal of Environment and Bio-Sciences 2019; 33(2): 243-250.
- Rao B.S, Venkateswaralu P. Physicochemical analysis of selected groundwater samples. Indian Journal of Environmental Protection 2000; 20(3): 161.
- Thangamalathi S, Anuradha V. Seasonal variations in physico-chemical parameters of seven different lakes in Chennai, Tamil Nadu, India. Journal of Environmental Science Toxicology and Food Technology 2018; 12(9): 11-17.
- Sooraj M.S.A. 2018. Water quality analysis in Velacherry lake, Chennai – a review. International Journal of Pure and Applied Mathematics 2018; 119(16): 4769-4774.
- Pokhriyal A, Uniyal D.P, Aswal J.S, Singh P, Dobhal R. Water quality assessment of drinking water sources of district Nainital in Uttarakhand. India. Journal of Environment and Biosciences 2019; 33(1), 67-76.
- Liu Y, Hou L, Bian W, Zhou B, Liang D, Li J. Turbidity in combined sewer sewage: an identification of stormwater detention tanks. International Journal of Environmental Research and Public Health 2020; 17(9): 3053.
CrossRef - Aravindkumar J, Saravanakumar K, Gokulakrishnan M, Indira B. Assessment of physico-chemical parameters of water at environmentally degraded Pallikaranai marsh area, Chennai, India. International Journal of Scientific and Engineering Research 2014; 5(7): 1067-1070.
- Shyamala R, Shanthi M, Lalitha P. Physicochemical analysis of borewell water samples of Telungupalayam area in Coimbatore district, Tamil Nadu, India. European Journal of Chemistry 2008; 5(4): 924-929.
CrossRef - Jameel A.A. Evaluation of drinking water quality in Tiruchirappalli, Tamil Nadu. Indian Journal of Environmental Health 2002; 44(2): 108-112.
- Buridi K.R, Gedala R.K. Study on determination of physicochemical parameters of ground water in industrial area of Pydibheemavaram, Vizianagaram district, Andhra Pradesh, India. Austin Journal of Public Health and Epidemiology 2014; 1(2): 1-3.
- Pradeep K.J, Chourasia L.P. Hydrogeological studies of upper Urmil river basin, Chhatarpur district, Central India. Ecology Environment and Conservation 2000; 6(2), 272-275.
- Soetan K.O, Olaiya C.O, Oyewole O.E. The importance of mineral elements for humans, domestic animals and plants: A review. African Journal of Food Science 2010; 4(5): 200-222.
- Matini L, Moutou JM, Kongo-Mantono M.S. Evaluation hydro-chimique des eaux souterraines en milieu urbain au Sud-Ouest de Brazzaville, Congo. Afrique Science 2009; 5(1): 82-98.
CrossRef - Kabour A, Heni A, Chebbah L, Sadek Y. Wastewater discharge impact on groundwater quality of Béchar city, southwestern Algeria: An anthropogenic activities mapping approach. Procedia Engineering 2012; 33: 242-247.
CrossRef - Benrabah S, Attoui B, Hannouche M. Characterization of groundwater quality destined for drinking water supply of Khenchela city (Eastern Algeria). Journal of Water and Land Development 2016; 30: 13-20.
CrossRef - Nas B, Berktay A. Groundwater contamination by nitrates in the city of Konya (Turkey): A GIS perspective. Journal of Environmental Management 2006; 79: 30-37.
CrossRef - Davie T. Fundamental of hydrology. Routledge, Taylor & Francis Group, London, New York 2003.
- Sagar S.S, Chavan R.P, Patil C.L, Shinde D.N, Kekane S.S. Physico-chemical parameters for testing of water-A review. International Journal of Chemical Studies 2015; 3(4), 24-28.
- Everett E.T. Fluoride’s effects on the formation of teeth and bones, and the influence of genetics. Journal of Dental Research 2011; 90(5): 552-560.
CrossRef - Meride Y, Ayenew B. Drinking water quality assessment and its effects on residents health in Wondo genet campus, Ethiopia. Environmental Systems Research 2016; 5: 1-7.
CrossRef - Sumathi M, Vasudevan N. Role of phosphate in eutrophication of water bodies and its remediation. Journal of Chennai Academy of Sciences 2019; 1: 65-86.
- Nyamangara J, Jeke N, Rurinda J. Long term nitrate and phosphate loading river water in the Upper Manyame catchment, Zimbabwe. Water SA 2013; 39(5): 637-642.
CrossRef - Williams M, Todd G.D, Roney N. Toxicological profile for manganese. U.S. Department of Health and Human Services. Public Health Service. Atlanta, Georgia 2012.






