Patterns of Phenological Characteristics of Important Tree Species of Kumaun Himalaya
Corresponding author Email: amitforestry26@gmail.com
DOI: http://dx.doi.org/10.12944/CWE.16.1.15
Copy the following to cite this article:
Mittal A, Tewari A, Singh N, Sharma S. Patterns of Phenological Characteristics of Important Tree Species of Kumaun Himalaya. Curr World Environ 2021;16(1). DOI:http://dx.doi.org/10.12944/CWE.16.1.15
Copy the following to cite this URL:
Mittal A, Tewari A, Singh N, Sharma S. Patterns of Phenological Characteristics of Important Tree Species of Kumaun Himalaya. Curr World Environ 2021;16(1). Available From : https://bit.ly/38KzhBR
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 22-01-2021 |
|---|---|
| Accepted: | 27-02-2021 |
| Reviewed by: | 
 Dr. R. K. Mathukia
Dr. R. K. Mathukia
|
| Second Review by: |

 Darwin H. Pangaribuan
Darwin H. Pangaribuan
|
| Final Approval by: | Dr. V.P. Tewari |
Introduction
The entire Himalaya region is highly vulnerable to the impacts of global warming and forest ecosystem can be seriously impacted in these changes. Phenological events of the plants are good indicators of climate differences1,2.Phenology involves precise documentation of timing and duration of different phenological events at species level, their interrelations and possible causal links between environmental variables and phenology3.The various phenological events such as leaf-expansion, abscission, flowering, bud-burst, fruiting, seed dispersal and germination of Himalayan species all take place in due season3,4. Phenological studies are as important to understand the species interactions and community function because each phenological events of each species occurs in its own calendar slot5. Fruiting must wait upon flowering; seed dispersal cannot precede fruiting even an individual flower undergoes a sequence of events 4,6.
A number of studies in different parts of the world indicate that global warming of last three decades has advanced by a few days several spring time activities such as leaf production flowering and fruiting in plants7,8. The observed change may be a positive sign because species are apparently adapting to changing climatic conditions, or they may be negative sign because they show that climate change is indeed impacting living systems9.A number of studies have convincingly demonstrated that plants are already responding to climate change with earlier leafing, flowering and leaf drop10,12. It is an important component for predicting how species will respond to global warming and increasing drought stress in recent scenario of changing climatic patterns13.The most significant ways by which trees can react and cope with rapid environmental change could be adjustments of phenological pattern, allowing trees to persist in their environment14,15.Phenological phases are closely linked with temperature, rainfall and photoperiod and adversely affect the pattern of phenology in same or different species on a small region.A number of evidenceshave been reported by various researches that phenophases of several species changed by changing climatic patterns. The present work focuses on the documentation of the phenological events and compared with earlier studies to find the shift the phenophases in last three decades and effect of climate change on phenological events ofsal, chir-pine and banj oak dominated forests in Nainital forest division of Kumaun Himalaya.
Material and Methods
The study sites were selected across an altitudinal transect located between 413 and 2345m elevation (between 290 18/and 290 24/ N and 790 19/and 79030/ E) in sal, chir-pine and banj oak dominated forests in Nainital forest division of Kumaun Himalaya. In the sal dominated forest Shorea robustaRox(Sal) and Mallotus philippinensis(Lam.)Muell.Arg (Rohini) in chir-pine dominated forest Pinus roxburghiisarg(Chirpine) and Myrica esculentaThumb (Kaphal) and in Oak dominated forest Quercus leucotrichophoraA.camus(Banj Oak) and Rhododendron arboreumWall (Buransh) were selected for detailed phenological observation (Table 1).
Table 1: Physiographic Features of selected Forests Studied Sites.
|
Site |
Study species |
Elevation(m) |
Aspect |
Latitude N |
Longitude E |
|
I |
S. robusta and M. philippinensis |
413-983 |
Level ground |
29018/35.1// 29019/9.5// |
079022/40.6// 079022/43.9// |
|
II |
P. roxburghii andM. esculenta |
1760-1810 |
South-East |
29023/15.1// 29023/18.5// |
079029/32.5// 079030/38.3// |
|
III |
Q. leucotrichophoraand R.arboreum |
1761-2345 |
North-East |
29023/16.0// 29023/42.1// |
079030/31.0// 079026/59.1// |
In sal forest the average annual precipitation was 1201mmandmean annual temperature was 23.40C with mean minimum temperature was 7.50C in the months of December and mean maximum temperature was 35.50C in the months of June. In oak and pine forest average annual precipitation was 2258mm of which two third occurred during rainy season (mid-June to mid-September. Mean annual temperature was 15.20C with mean minimum temperature was 4.60C in the months of January and mean maximum temperature was 25.90C in the months of June.
Irrespective of site 30 individual trees of each selected species (one dominant and one under canopy species) were marked for S.robusta,M. philippinensis, P. roxburghii,M. esculenta,Q. leucotrichophora and R. arboreum over a 2.0 ha area. The phenological observations were made at 15 days interval during low activity period and weekly in the periods of high phenological activity4,16. Phenological records were made for four phenophases, viz., leafing, leaf drop, flowering and seed fall for all studied species for a two-year period and compared with earlier studies to find the shift in phenological events in last three decades.
Results and Discussion
Shorea Robusta
Across all the sites the leaf fall in S. robusta started from March 2nd week and was complete by the end of April. Flower bud break started from March 2nd week and flowering was in <10% trees after 2-3 days of flower bud break. Flowering had peaked in the 4th week of March (75% trees had flowered). New leafing started after one week of floral bud opening and by the April end trees had maximum new leafing (95%). However, in seedlings and saplings it continued till July end. Seed fall started from June first week and almost all fruits had fallen after the torrential rain in the third week of June (Fig.1 and 2).
Mallotus Philippinensis
In this species the leaf fall started in the 2nd week of June and was complete in August 2nd week. New leaves started appearing from May 3rd week and leafing was completed in August 1stweek. However, in saplings and seedlings new leaves appeared after July during August and September. Flowering started from September end and was completed in the mid of November. Fruiting commenced from the beginning of December and seed fall was complete by the 3rd week of April (Fig.1 and 2).
Pinus Roxburghii
Needle bud enlargement started from February end and was complete by the 2nd week of March. Needles had attained their maximum length ranging between 14.3cm to 17.1cm by May end. Leaf fall started from March last week and was over by May 2nd week. Seed dispersal commenced from April 2nd week and was completed by June 2ndweek(Fig. 1 and 2).
Myrica Esculenta
Leafing started from April 2nd week and was completed by May end when >95% trees and saplings had leafed. Leaf fall started from June 2nd week and continued till 2nd week of July. Seed fall was observed from April end and was completed by 3rd week of May only occasional trees had fruits. Only a few trees (10%) of M. esculenta had seeds, and it may be concluded that year as a lean seed year. Male flowers appear from August end and flowering was complete by October end. Maximum flowering was observed between August end and September 2nd week when 80% trees had flowers (Fig.1 and 2).
Quercus Leucotrichophora
Seed fall commenced from 2nd week of November and seed fall was complete (85%) by January end. Bud bursting started from February end and was completed by March end across all the sites. Leafing started in the 1stweek of March and was completed in the 1stweek of April in trees. Leaf fall started simultaneously with bud bursting and continued till April end. Acorn appeared from March 1stweekand continued to appear till 2ndweek of April. Seedlings and saplings showed late bud opening, leafing and leaf fall compared to matured trees. Bud bursting was earlier at the disturbed sites(Fig.1 and 2).
Rhododendron Arboreum
Flower bud bursting and flowering started from 1stweek of February (<5% trees had flowers). However, occasional trees started flowering from February 1stweek. Flowering peaked in 1st week of April (>75% tree had flowered) and was completed by the end of May. New leaves appear after the completion of flowering from May 3rdweek and was completed by the end of rainy season. Leaf fall took place round the year but was maximum during the summer months (May-June). Seed dispersal started from February end and all the capsules had opened by mid-March. Leaf longevity of this species is more than 16 months (Fig.1 and 2).
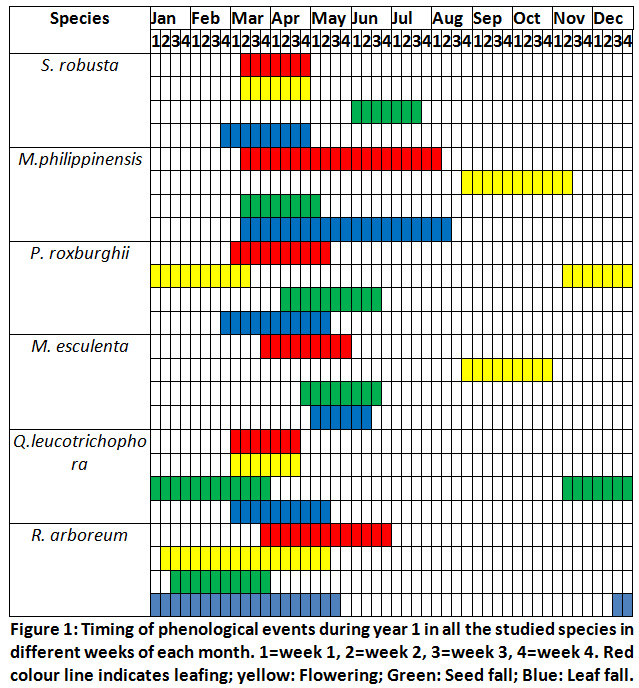 |
Figure 1: Timing of Phenological Events during Year 1. Click here to view Figure |
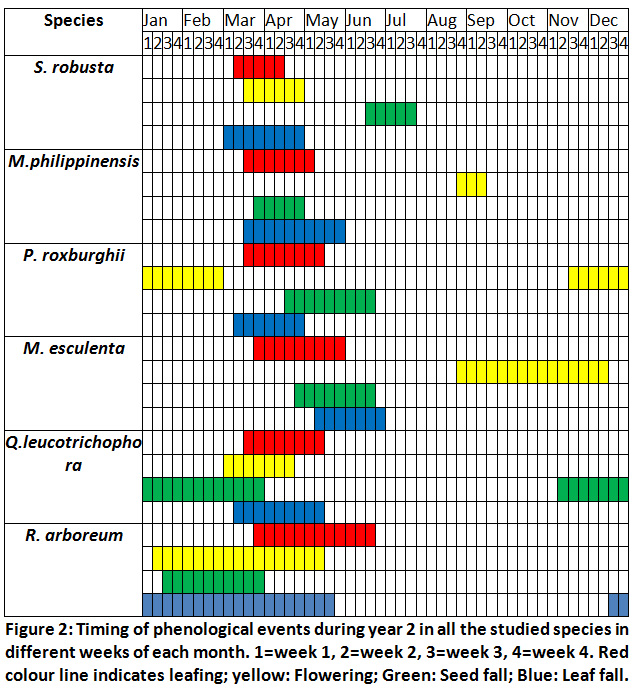 |
Figure 2: Timing of Phenological Events during Year 2. Click here to view Figure |
We compared the timing of phenological events of present study with the earlier studies of 17,18.In S. robusta when we compared the timing of flowering initiation and completion with earlier studies, we do not find any significant shifts in timing of these phenological events17,18. There was no perceptible change in the initiation of leaf drop and leaf fall completion in comparison18. However, the period of leaf flushing has become small by approximately a month in comparison to earlier studies (Table 2). In M. phillippinensis the period of leafing has been reduced by approximately 4-6 week. The period of leaf drop and leaf flushing were similar to17. In P. roxburghii the time of flowering initiation has become earlier by 4-6 weeks. Similarly, commencement of needle drop was also earlier by 4-5 weeks in comparison to earlier studies (Table 2). In M. esculentathe timing of flowering initiation and completion showed no change in comparison to earlier studies. Leaf flushing initiation was earlier by 4 weeks in both the years whereas leaf drop initiation was delayed by 2 week and completion was same incomparison to earlier studies (Table 2). In Q. leucotrichophora there appear to be no major changes in the timing of flowering and new leaf flushing; however, the period of leaf drop has become extended by 2-3 weeks in comparison to earlier studies (Table 2). In R. arboreum it has reported flowering initiation in January extending up to April17. Earlier researcher has given more restricted period of flowering February-March for the species18. In our study flowering commenced from January II week and continued up to May II week in both the years showing more extended flowering period. The period of leaf drop also shows an extended period coupled with leaf flushing (Table 2).
Table 2: Comparison of Changes in Timing of Leaf Drop, Leafing and Flowering in Studied Species with Earlier Studies.
|
Species |
Leaf drop |
Leaf flush |
Flowering |
Source |
|
S. robusta |
Mar-June |
Mar-July |
Apr-May |
Ralhan, 1985 |
|
Feb-Apr |
Mar-June |
Feb-Apr |
Negi, 1989 |
|
|
Feb IV-Apr IV week |
Mar II-Apr IV week |
Mar II-Apr IV week |
Yr 1 present study |
|
|
Mar I-Apr IV week |
Mar III-May I week |
Aug IV-Sept II week |
Yr 2 present study |
|
|
M.philippinensis |
Mar II-Aug II |
Apr-May |
Sep-Oct |
Ralhan, 1985 |
|
Mar II-Aug II week |
Mar II-Aug I week |
Aug IV-Nov II week |
Yr 1 present study |
|
|
Mar III-May IVweek |
Mar III-May Iweek |
Aug IV-Sept IIweek |
Yr 2 present study |
|
|
P.roxburghii |
May-June |
Feb-Apr |
Feb-Mar |
Ralhan, 1985 |
|
Apr-June |
Mar-Apr |
Jan-Feb |
Negi, 1989 |
|
|
Feb IV-May II week |
Mar I-May II week |
Nov II-Mar II week |
Yr 1 present study |
|
|
Mar II-Apr IV week |
Mar III-May II week |
Nov III-Feb IV week |
Yr 2 present study |
|
|
M.esculenta |
Mar-May |
Apr-May |
Feb-Mar |
Negi, 1989 |
|
Apr II-May II week |
Mar IV-May IV week |
Feb IV-Mar IV week |
Yr 1 present study |
|
|
Apr II-May II week |
Mar IV-May IV week |
Feb IV-Mar IV week |
Yr 2 present study |
|
|
Q.leucotrichophora |
Apr-May |
Mar-Aug |
Mar-Apr |
Ralhan, 1985 |
|
Feb-Apr |
Mar-Apr |
Mar-Apr |
Negi, 1989 |
|
|
Mar I-May II week |
Mar I-Apr III week |
Mar I-Apr III week |
Yr 1 present study |
|
|
Mar II-May II week |
Mar III-May II week |
Mar I-Apr III week |
Yr 2 present study |
|
|
R.arboreum |
Jan-Dec |
Mar-Apr |
Jan-Apr |
Ralhan, 1985 |
|
Feb-Apr |
Apr-May |
Feb-Mar |
Negi, 1989 |
|
|
Dec III-May III week |
Mar IV-June IV week |
Jan II-May II week |
Yr 1 present study |
|
|
Dec III-May III week |
March IV-June III week |
Jan II-May II week |
Yr 2 present study |
Conclusion
It is apparent from the present study that due to climatic irregularities and temperaturerise the role of temperature would become paramount in controlling/shifting of the phenological events. Many species shifted their flowering time across the worldwide. Global warming could be a primary cause for these changes some other factors also responsible for these changes such as precipitation pattern, soil and water stress, moisture condition andphotoperiod that would be useful to better understand spatial patterns in the sensitivity of phenological responses to temperature. Microclimatic condition alsoresponsible for controlling/shifting the phenological patterns of same or different species.Hence, more detailed investigations at the local level are required to examine the influence of these events in future studies.
Acknowledgements
The authors are thankful to the Director, Graphic Era Hill University, Bhimtal Campus and Head, Department of Forestry and Environmental Science, Kumaun University, Nainital for providing facilities in the department.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The author(s) declares no conflict of interest.
References
- Poudyal K. Plant water relations and phenological shifts in response to drought in Castanopsis indica at Phulckowki Hill, Kathmandu, Nepal. Journal of Biological Science Opinion. 2014; 2(1):70-76.
CrossRef - Singh N, Tewari A, Shah S, Mittal A. Water Relations and Phenological Events of two Treeline Rhododendron species in Indian Western Himalaya. Sylwan. 2019; 163(10):164-176.
- Singh N, Mittal A. Response of phenological events of Aesculus indicaColebr. to climate change along an altitudinal gradient in Kumaun Himalaya, Uttarakhand. International Journal of Environment. 2018; 8(1):1-16.
- Singh N, Ram J, Tewari A, Yadav R P. Phenological events along the elevation gradient and effect of climate change of Rhododendron arboreum Sm. in Kumaun Himalaya. Curr Sci. 2015; 108(1):106-110.
CrossRef - Singh N. Flowering phenology of tree Rhododendron arboreum along an elevation gradient in different sites of Kumaun Himalayas. International Journals of Science and Nature. 2014; 5(3):572-576.
- Fenner M. The phenology of growth and reproduction in plants. Perspectives in Plant Ecology, Evolution and Systematic. 1998; 1(1):78-91.
CrossRef - Hughes L. Biological consequences of global warming: Is the signal already apparent?Trends in Ecology and Evolution. 2000; 15:56-61.
CrossRef - Mittal A, Singh N, Tewari A.Quantitative analysis and regeneration status of forest trees species in Kumaun Central Himalaya. Indian Journal of Ecology. 2020; 47(2):507-513.
- Visser ME, Both C. Shifts in phenology due to global climate change: The need for a yardstick. Proceedings of the Royal Society B: Biological Sciences. 2006; 272(1581):2561-9.
CrossRef - Negi G C S, Singh S P. Leaf growth pattern in evergreen and deciduous species of the Central Himalaya, India. International Journal of Biometeorology. 1992; 36(4):233-242.
CrossRef - Miller-Rushing A, Primack R. Global warming and flowering times in Thoreau’s Concord: a community perspective. Ecology. 2008a; 89(2):332-341.
CrossRef - Tewari A, Shah S, Singh N, Mittal A. Treeline species in Western Himalaya are not water stressed: a comparison with low elevation species. Trop Ecol. 2018; 59(2):313–325.
- Mittal A, Singh N, Tewari A, Shah S.Cone maturation timing and seed germination in Pinus roxburghii (Serg.) in the central Himalayan region of Uttarakhand, India. Ecol Envi &Conse. 2020;26:286-290.
- Pigliucci M, Murren CJ, Schlichting CD. Phenotypic plasticity and evolution by genetic assimilation. Journal of Experimental Biology. 2006; 209:2362-2367.
CrossRef - Malfasi F, Cannone N. Climate warming persistence triggered tree ingression after shrub encroachment in a High Alpine Tundra.Ecosystems, 2020; 23:1657–1675.
CrossRef - Singh N, Tewari A, Shah S.Tree regeneration pattern and size class distribution in anthropogenically disturbed sub-alpine treeline areas of Indian Western Himalaya.International Journal of Scientific and Technology Research. 2019; 8(8):537-546.
- Ralhan P K. The physiology of plant in forest ecosystem of Kumaun Himalaya. Ph.D Thesis, Kumaun University, Nainital, 1985.
- Negi G C S. Phenology and Nutrient dynamics of tree leaves in Kumaun Himalaya. Ph.D Thesis, Kumaun University, Nainital, 1989.






