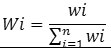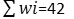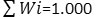Hydro chemical Assessment of Ground Water in North-Eastern Region of India: A Case Study of Western Suburb of Jorhat Town of Assam, India
Paran Jyoti Kalita1,2
 , Champa Gogoi1,3
, Champa Gogoi1,3
 , Sausthov Maunash Bhattacharyya4
, Sausthov Maunash Bhattacharyya4
 and Rajib Lochan Goswamee1
*
and Rajib Lochan Goswamee1
*

1
Advanced Materials Group, Materials Science and amp; Technology Division,
CSIR-North East Institute of Science and amp; Technology,
Jorhat,
Assam,
India
2
Department of Chemistry,
Gauhati University,
Guwahati,
Assam,
India
3
Department of Chemistry,
CNB College, Bokakhat,
Golaghat,
Assam,
India
4
Geo Sciences and amp; Technology Division,
CSIR-North East Institute of Science and amp; Technology,
Jorhat,
Assam,
India
DOI: http://dx.doi.org/10.12944/CWE.16.1.04
Copy the following to cite this article:
Kalita P. J, Gogoi C, Bhattacharyya S. M, Goswamee R. L. Hydro chemical Assessment of Ground Water in North-Eastern Region of India: A Case Study of Western Suburb of Jorhat Town of Assam, India. Curr World Environ 2021;16(1). DOI:http://dx.doi.org/10.12944/CWE.16.1.04
Copy the following to cite this URL:
Kalita P. J, Gogoi C, Bhattacharyya S. M, Goswamee R. L. Hydro chemical Assessment of Ground Water in North-Eastern Region of India: A Case Study of Western Suburb of Jorhat Town of Assam, India. Curr World Environ 2021;16(1). Available Form : https://bit.ly/2XTNI0I
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2020-06-08 |
|---|---|
| Accepted: | 2021-01-01 |
| Reviewed by: | 
 B. Purandara
B. Purandara
|
| Second Review by: |

 Jay Balkhande
Jay Balkhande
|
| Final Approval by: | Dr. Gopal Krishan |
Introduction
In this world, clean and safe water for drinking is one of the important fundamental needs for the survival of human being on earth. 1 In India accessibility of drinking water is a challenging task. In recent years, resources of fresh water system have been affected by the rapid population growth as well as development of economic system. 2, 3 All over the world, water plays the key role in the transmission of different water borne diseases and hence maintenance of the quality of drinking water has become a universal health concern.4 Throughout the world, natural potable water resources are exceedingly decreasing due to disturbances caused by human race. In addition to this, occurrence of various types of pollutants, including the heavy metals, introduced to water through natural or anthropogenic activities causes toxic and harmful effects to the individuals and the environment.5 In India, arsenic and fluoride are such pollutants of groundwater which have been identified as a major problem and therefore, mitigation measures are in progress to solve these issues. Presence of many inorganic anions and oxy-anions in water such as AsO4- , AsO3-, F-, Cl-, HCO3-, SO42-, PO43-, NO3- in elevated amounts can also deteriorate the quality of water. So, assessment of these ions in water is of utmost importance for safety of people and environment. Apart from chemical composition, the accessibility of safe water with high quality index for its use in agriculture, irrigation, and drinking purposes is important for disease free high quality life. In India, ground water is the most reliable source for drinking, agriculture, and irrigation.6 Most of the people in India, rely on ground water supply for drinking as well as for irrigation.7,8 Therefore, the public health-related issues are of immense concern to the government. In rural areas, people are more dependent on ground water for drinking, and hence the quality evaluation of ground water is an essential concern for the heath of rural people. 9, 10 To know about the usability of a water source of particular region, the water quality assessment is highly essential for the region.
In our present study, 69 number of ground water samples from Saroocharai and Charaibahi Mouza (a unit of cluster of villages made for land revenue collection) in western sub urban fringe of Jorhat town of Jorhat District, Assam were collected for evaluation of quality to know about their usability for irrigation and drinking. The area is selected for study due to its importance as one of the important academic, business and strategic centre of North East India. The area under investigation has strong presence of both tea and oil industry. It is a mix of urban, urban over growth, semi urban and rural localities where most of the people are dependent on ground water for drinking and domestic uses. The chosen Charaibahi Mouza have important research and academic institutions like two national laboratories, two universities, minimum five hospitals, three medical education institutes, one management institute, a historical government prison, airport, important defence and police establishments, several high schools etc. On the hand the Saroocharai Mouza situated to the north and separated from the adjoining Charaibahi Mouza by national highway no 37 is predominantly an agricultural area with some large tea garden and a big ancient paddy field called Malow Pathar as the mainstay of the agricultural background. With increasing population, urbanisation, and real estate development, both these areas are growing areas for future denser settlement development as well as immediate agricultural, dairy, and animal products source zones.
To know about the usability of ground water of these two areas for drinking, various physicochemical parameters were determined and checked their permissibility limit with the available standards. WQI of the each water samples were evaluated in order to know the quality of GW for drinking. Also, various parameters such as Sodium Adsorption Ratio (SAR), Sodium Percentage, Kelly’s Ratio (KR), Magnesium Ratio (MR), and Corrosivity Ratio (CR), Residual Sodium Carbonate (RSC) were evaluated to check the quality of the ground water for irrigation.
Materials and Methods
Study Area
The sites of Saroocharai and Charaibahi Mouza (latitude 26°43.2150 -26°48.2070' N and longitude 94°5.2740'- 94°11.5500'E) of western part of Jorhat town of Assam, India as depicted in Figure 1 were selected for the collection of GW sample. The climate of Jorhat District is sub-tropical humid (wet) with dry winters. The average temperature in winter season is 16.6°C and in summer it is 28.9°C. However, the minimum temperature in winter can come down to 9.9°C and in summer maximum temperature can go up to 36.8°C. The annual rain fall in Jorhat District in 2017 was 2107 mm with a monthly average rain fall of 176 mm. The northern part of the investigated area is situated on the flood plains of the western banks of Bhogdoi river, a southern bank tributary of Brahmaputra River. The top soil quality of the area is mostly alluvial soil. The water being exploited for drinking and domestic purposes are mainly from shallow tube wells. In our present study, assessment of ground water was done by collecting water from these wells.
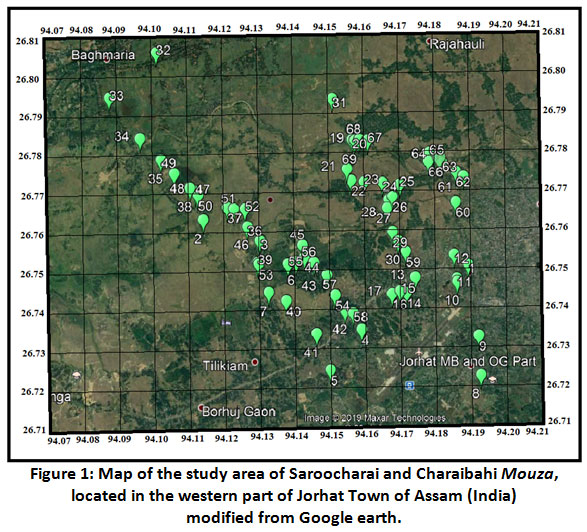 |
Figure 1: Map of the study area of Saroocharai and Charaibahi Mouza, located in the western part of Jorhat Town of Assam (India) modified from Google earth. Click here to view Figure |
Ground Water Sampling and Analysis
Total sixty nine ground water (GW) samples were collected from various locations of Saroocharai and Charaibahi Mouza of western part of Jorhat town of Jorhat district during pre monsoon period (May to June) and during post monsoon period (November to December) in the year 2017. Hand pumps or bore well water sources were selected for ground water sample collection. Polythene bottles of 1L capacity were used for sample collection after washing with 8 M nitric acid and then with distilled water for several times. The bottles were rinsed with sample water for three times before sample collection. 11 The water samples taken for arsenic (As) determination were maintained at pH (<2) and was kept at 4°C in laboratory refrigerator (Remi RRF-250). The water samples were taken for determination of physicochemical parameters like pH, total hardness (denoted as TH), total alkalinity (denoted as TA), crucial anions such as HCO3-, Cl-, NO3-, and SO42-; component such as F-, PO43-, primary cations- Na+, and K+, Mg2+, Ca2+; and heavy metal like iron were kept at 4°C in a laboratory refrigerator (Remi RRF-250). Flame photometer (Systronic Flame photometer 128) was used to determine Na+ and K+, the anions SO42-, PO43-, NO3-, Cl- were determined by using an 881Compact IC pro Metrohm ion chromatograph (Made Switzerland). Total dissolved solid (denoted as TDS) was determined with TDS meter, (EUTECH Instruments). Ca2+, Mg2+ and HCO3- were determined by titrimetric method (APHA 1988).11 The concentration of iron was measured by 1, 10-Phenanthroline method using an UV visible spectrophotometer (Shimadzu 05500) at 510 nm (APHA 1988). 11 Arsenic concentrations were determined in atomic absorption spectrometer, Make: Perkin Elmer, Model: AAnalyst-700. Orion 4 star (pH/ISE) ion selective electrode was used to detect the presence of fluoride (F-) in water samples after calibration with 0.1, 1, 10 mgL-1 standard fluoride solutions. Conductivity meter 306 (Made Systronic) was used to measure electrical conductivity (EC). pH was measured at the time of collection of water sample by using a hand pH meter (Model: pHep made by Hanna Instruments)
Statistical analysis was done by using Statistical Package for Social Sciences (IBM SPSS statistics Version 25). The software was used to determine descriptive statistics and to evaluate the correlation among different parameters. Origin software was used to draw different plots. The Longitude and Latitude of the regions under the study are listed in Table 1.
Table 1: Description of Sample Collection Site
|
Sample Code No |
Latitude (deg N) |
Longitude (deg E) |
Sample Code No |
Latitude (deg N) |
Longitude (deg E) |
|
1 |
26.74821 |
94.18938 |
36 |
26.75860 |
94.12673 |
|
2 |
26.76056 |
94.11420 |
37 |
26.76361 |
94.12103 |
|
3 |
26.75510 |
94.13041 |
38 |
26.76871 |
94.11051 |
|
4 |
26.73218 |
94.15880 |
39 |
26.74958 |
94.12948 |
|
5 |
26.72208 |
94.14976 |
40 |
26.73975 |
94.13748 |
|
6 |
26.74966 |
94.14051 |
41 |
26.73130 |
94.14598 |
|
7 |
26.74190 |
94.13253 |
42 |
26.73701 |
94.15416 |
|
8 |
26.72025 |
94.19249 |
43 |
26.74611 |
94.14916 |
|
9 |
26.73021 |
94.19208 |
44 |
26.75073 |
94.14288 |
|
10 |
26.74395 |
94.18620 |
45 |
26.75371 |
94.14233 |
|
11 |
26.74478 |
94.18608 |
46 |
26.75505 |
94.13018 |
|
12 |
26.75078 |
94.18540 |
47 |
26.76771 |
94.11211 |
|
13 |
26.74521 |
94.17426 |
48 |
26.76796 |
94.11208 |
|
14 |
26.74128 |
94.17175 |
49 |
26.77248 |
94.10608 |
|
15 |
26.74181 |
94.16998 |
50 |
26.76685 |
94.11278 |
|
16 |
26.74108 |
94.16759 |
51 |
26.76323 |
94.12289 |
|
17 |
26.74110 |
94.16766 |
52 |
26.76320 |
94.12609 |
|
18 |
26.74108 |
94.16764 |
53 |
26.74916 |
94.12973 |
|
19 |
26.780216 |
94.15768 |
54 |
26.74096 |
94.15141 |
|
20 |
26.78040 |
94.15693 |
55 |
26.74906 |
94.13813 |
|
21 |
26.77300 |
94.15530 |
56 |
26.74936 |
94.14550 |
|
22 |
26.77006 |
94.15643 |
57 |
26.74096 |
94.15141 |
|
23 |
26.76958 |
94.16011 |
58 |
26.73640 |
94.15648 |
|
24 |
26.76936 |
94.16540 |
59 |
26.75175 |
94.17166 |
|
25 |
26.76888 |
94.17023 |
60 |
26.76418 |
94.18610 |
|
26 |
26.76578 |
94.16814 |
61 |
26.77068 |
94.18840 |
|
27 |
26.76496 |
94.16710 |
62 |
26.77191 |
94.18630 |
|
28 |
26.76288 |
94.16658 |
63 |
26.77413 |
94.18246 |
|
29 |
26.75678 |
94.16801 |
64 |
26.77486 |
94.18160 |
|
30 |
26.75445 |
94.16963 |
65 |
26.77686 |
94.17886 |
|
31 |
26.79096 |
94.15141 |
66 |
26.77460 |
94.17853 |
|
32 |
26.80345 |
94.10141 |
67 |
26.78020 |
94.16130 |
|
33 |
26.79201 |
94.08790 |
68 |
26.78040 |
94.15891 |
|
34 |
26.78151 |
94.09649 |
69 |
26.78020 |
94.15768 |
|
35 |
26.77600 |
94.10240 |
|
|
|
Results and Discussion
Assessment of Ground Water for Drinking
The evaluation of different physicochemical parameters of ground water samples is very much essential to check their suitability for drinking. Therefore, the parameters were evaluated and the statistical summary is listed in Table 2.
Table 2: Physicochemical characteristic of collected water samples in Pre monsoon (pr-) & post monsoon (pos-) season.
|
Parameters |
Unit |
Minimum |
Maximum |
Mean |
SD |
WHO permissible Limit |
||||
|
Pr- |
Pos- |
Pr- |
Pos- |
Pr- |
Pos- |
Pr- |
Pos- |
|
||
|
pH |
- |
5.2 |
5.5 |
7.7 |
7.2 |
6.5 |
6.3 |
0.611 |
0.433 |
6.5-8.5 |
|
EC |
µscm-1 |
155.8 |
156.7 |
224.5 |
222.4 |
179.1 |
181.1 |
16.9 |
15.8 |
1000-2000 |
|
Hardness |
mgL-1 |
39.8 |
40.8 |
118.4 |
120.2 |
66.1 |
68.7 |
19.8 |
33.67 |
500 |
|
TDS |
mgL-1 |
162.9 |
165.6 |
212.9 |
219.7 |
180.5 |
184.5 |
13.0 |
12.8 |
1000 |
|
Ca2+ |
mgL-1 |
6.57 |
7.13 |
18.35 |
18.59 |
10.98 |
11.16 |
2.95 |
2.99 |
100 |
|
Mg2+ |
mgL-1 |
5.36 |
5.60 |
17.69 |
17.98 |
9.43 |
9.94 |
3.12 |
3.1 |
150 |
|
Na+ |
mgL-1 |
2.1 |
1.1 |
11.3 |
14.6 |
5.7 |
4.5 |
2.34 |
2.68 |
200 |
|
K+ |
mgL-1 |
2.00 |
3.1 |
14.5 |
13.5 |
9.4 |
9.5 |
3.46 |
2.64 |
12 |
|
Fe |
mgL-1 |
0.6 |
0.6 |
5.9 |
5.4 |
3.1 |
3.3 |
1.27 |
1.2 |
0.3 |
|
Cl- |
mgL-1 |
1.2 |
3.26 |
8.0 |
9.45 |
6.69 |
6.35 |
1.42 |
1.098 |
250 |
|
SO42- |
mgL-1 |
0.138 |
0.198 |
0.747 |
0.946 |
0.232 |
0.287 |
0.0844 |
0.101 |
400 |
|
PO43- |
mgL-1 |
0.18 |
0.1 |
2.58 |
1.33 |
0.644 |
0.453 |
0.438 |
0.272 |
0.10 |
|
F- |
mgL-1 |
BDL |
BDL |
0.650 |
0.710 |
0.303 |
0.380 |
0.135 |
0.146 |
1.5 |
|
NO3- |
mgL-1 |
0.18 |
0.12 |
11.95 |
10.54 |
2.77 |
2.30 |
2.54 |
2.31 |
50 |
|
As |
mgL-1 |
BDL |
BDL |
0.07 |
0.056 |
0.007 |
0.0047 |
0.014 |
0.009 |
0.01 |
|
TA |
mg/L |
92.2 |
100 |
108.9 |
135 |
95.6 |
107.8 |
3.05 |
7.4 |
200 |
The collected ground water (GW) water samples have pH ranges from 5.2 to 7.7 in pre monsoon seasons (mean 6.5) and from 5.5 to 7.2 in post monsoon season (mean 6.3). From the pH data it was found that in pre-monsoon season 20.29 % of water samples and in the post-monsoon season 25 % of water samples have acidic pH. The percentage of GW water samples having alkaline pH in pre monsoon season was found to be 33.33% and in post monsoon season it was 8.83%. However, all the GW samples had pH within permitted range (6.5-8.5) of World Health Organisation (WHO 2011). 12 On the basis of pH values the water sources were found to be appropriate for drinking as well as for irrigation. The presence of slightly acidity in some GW can be attributed to the dissolution of atmospheric carbon dioxide and organic acids like fulvic and humic acid, which are formed from the decay and following leaching of plant materials.13Use of ammonium sulphates and super phosphate of lime as fertilizers may also lower the pH since the area is full of agricultural land.13 On the basis of pH value the water samples were classified in four pH zones as shown in Table 3.
Table 3: Samples Categorization on the Basis of pH.
|
Range |
% of water sources |
|
|
Ground water |
||
|
Pre-monsoon |
Post-monsoon |
|
|
<6.0 |
20.3 |
25 |
|
6.0-6.5 |
30.4 |
42.6 |
|
6.5-7.0 |
15.9 |
23.5 |
|
>7.0 |
33.4 |
8.8 |
|
Total |
100 |
100 |
The TDS values of the GW samples was found to be in the range of 162.9-212.9 mgL-1 (Mean 180.5 mgL-1) in the pre-monsoon season and 165.6-219.7 mgL-1 (mean 184.5 mgL-1) in the post-monsoon season. All the GW samples were found to have TDS value below the World Health Organisation (1984) limit. 14 As per the threefold classification proposed by Davis and Dewiest (1966), all the water samples could be considered as domestic category (TDS value < 500 mgL-1) as shown in Table 4. 15
Table 4: Samples Categorization on the basis of Total Dissolved Solid (mgL-1).
|
Range |
Water category |
% of water sources |
|
|
Ground water |
|||
|
Pre-monsoon |
Post-monsoon |
||
|
<300 |
Excellent |
100 |
100 |
|
300-600 |
Good |
0 |
0 |
|
> |
Poor and Unacceptable |
0 |
0 |
|
Total |
|
100 |
100 |
The Hardness (as CaCO3) of the GW samples were found to be in the range of 39.8-118.4 mgL-1(mean value 66.1 mgL-1) in pre-monsoon season and 40.8-120.2 mgL-1 (mean value 68.7 mgL-1) in the post-monsoon season. However, all the ground water samples were found to have hardness values below the permissible limit of World Health Organisation (1984), which is 500 mgL-1. 14 According to Durfor and Becker (1964) classifications, the water samples can be classified into four categories as shown in Table 5. 16
Table 5: Samples Categorization on the basis of Total Hardness (mgL-1)
|
Class |
Hardness |
% water sources |
|
|
Ground water |
|||
|
Pre-monsoon |
Post-monsoon |
||
|
Soft |
0-60 |
50.7 |
42 |
|
Reasonably hard |
61-120 |
49.3 |
58 |
|
Hard |
121-180 |
0 |
0 |
|
Very Hard |
>180 |
0 |
0 |
|
Total |
|
100 |
100 |
The acid neutralising capacity of water is expressed in terms of alkalinity. 17 Alkalinity of ground water originates mainly from carbonates and bicarbonates. 18 The acceptable limit for alkalinity is 200 mgL-1 and in the absence of other alternate source alkalinity up to 600 mgL-1 could be accepted for drinking purpose (IS 10500-1991). 19 Carbonate alkalinity in the studied samples were absent which is due to the fact that carbonate anion is generally exist in water at pH more than 8.3.13 The total alkalinity in the studied samples arises from the presence of bicarbonates. The alkalinity of studied water samples were found in the range from 92.2-108.9 mgL-1 (mean 95.6mgL-1 ) in the pre-monsoon period and 100.0-135.0 mgL-1 (mean 107.8 mgL-1 ) in the post-monsoon period.
The concentration of ionised substances in water was measured in terms of Electrical Conductivity (EC) of water. 8, 20 In our present study, the EC was found to be varied in the range 155.8 µscm-1 to 224.5 µscm-1 (mean179.1µscm-1) in the pre monsoon and 156.7 µscm-1 to 222.4 µscm-1 (mean 181.1 µscm-1) in the post monsoon period.
The Ca2+ in the collected GW samples were found to lie in the range of 6.57-18.35 mgL-1 (mean 10.98 mgL-1) in pre monsoon period and 7.13-18.59 mgL-1 (mean 11.16 mgL-1) in post monsoon period. Similarly, the Mg2+ were found to be in the range of 5.36-17.69 mgL-1 (mean 9.43 mgL-1) and 5.60 -17.98 mgL-1 (mean 9.94 mgL-1) in the pre and post-monsoon periods respectively. In the two seasons all the ground water samples have concentration of Ca2+ and Mg2+ within the desirable limit set by BIS (2004) which is 75 mgL-1 for Ca2+ and 30 mgL-1 for Mg2+.
The amount of Na+ in the GW samples were found in the range of 2.1-11.3 mgL-1 (mean 5.7 mgL-1) in the pre monsoon season and 1.1-14.6 mgL-1 (mean 4.5 mgL-1) in the post monsoon period, respectively. The K+ content of ground water samples were found to be in the range of 2.0-14.5 mgL-1 (mean 9.4 mgL-1) in the pre-monsoon period and 3.1-13.5 mgL-1 (mean 9.5) in the post-monsoon period respectively. From the collected data it was found that 23.5 % of ground water samples in pre-monsoon season and 11.8 % GW samples in the post-monsoon period have slightly higher value of K+ than the desirable limit of 12 mgL-1. WHO (2011) and BIS (2004) recommended value for Na+ is 200 mgL-1 and K+ is 12 mgL-1 in drinking water. However, for all the samples, the Na+ is found to be within the recommended value of 200 mgL-1(WHO 2011, BIS 2004). From the above results for Na+ and K+, it may be stated that the water quality of the ground water in the studied area are suitable for consumption in domestic and drinking purposes.
Amount of iron content in the GW samples were found to be in the range of 0.6-5.9 mgL-1 (mean 3.1 mgL-1) in the pre-monsoon period and 0.6-5.4 mgL-1 (mean 3.3 mgL-1) in the post-monsoon season respectively. In both the seasons GW has iron content more than the permissible limit of 0.3 mgL-1 (IS 10500: 2012). 21The quality of water samples based on iron content is listed in Table 6. It is already reported that dissolution of iron is about 105 times greater at pH 6 than at pH 8.5. 13 Since our study area is acidic (Average pH <7), it is supposed that when the water percolates down it dissolves large amount of iron from soil.
Table 6: Samples Categorization on the basis of Iron content
|
Range |
% water sources |
|
|
Ground water |
||
|
Pre-monsoon |
Post-monsoon |
|
|
<0.3 mgL-1 |
0 |
0 |
|
0.3-1.5 |
8.7 |
11.76 |
|
1.5-5.0 |
81.2 |
82.35 |
|
>5.0 |
10.1 |
5.88 |
|
Total |
100 |
100 |
GW chloride anion was found to be varying in a wide range of values from 1.2-8.0 mgL-1 (mean 6.69 mgL-1) in the pre monsoon season and from 3.26-9.45 mgL-1 (mean 6.35 mgL-1) in the post-monsoon seasons. The studied water samples have low chloride content in both the seasons which may be due to the fact that there is no industrial activities and also rate of seepage of domestic and agricultural wastes in to water bodies is very low in the study area.22 Every samples were found to have chloride content within the permitted limit of WHO (600 mgL-1) and therefore, suitable for drinking and domestic uses.
The range of SO42- content in the studied GW samples were found to fall in the range of 0.138-0.747 mgL-1 (mean 0.232 mgL-1) in pre-monsoon season and 0.198-0.946 mgL-1 (mean 0.287 mgL-1 ) in post-monsoon season. All the water sources in both seasons have sulphate content within the permitted limit (200 mgL-1, WHO 2004) for drinking and domestic purposes.
The GW samples were found to have NO3- in the range of 0.18-11.95 mgL-1 (mean 2.77 mgL-1) in the pre-monsoon period and 0.12-10.54 mgL-1 (mean 2.30 mgL-1) in the post-monsoon season respectively. However, all the samples in both the seasons were found to have NO3- content less than the permitted value of WHO (2004) which is 50 mgL-1 for drinking water. High NO3- content may cause many health related issues such as methemoglobinemia or blue baby syndrome and cause to develop gastric an intestinal cancer. 23 The NO3- content in GW samples of our study area was found to be very low amount to cause such situation.
The concentrations of PO33- in our studied samples were found to fall in the range of 0.18-2.58 mgL-1 (mean 0.644 mgL-1) in the pre-monsoon period and 0.1-1.3 mgL-1 (mean 0.453mgL-1) in the post-monsoon period. However, the WHO permissible limit for PO43- is 0.1 mgL-1 (WHO 2004). In GW water samples of both seasons the PO43- contents are slightly higher than the permissible limit of drinking water. Weathering of rocks containing phosphate and percolation of agricultural runoff carrying residual fertilizers may be the reason of high PO43- contents in GW. 13
The fluoride (F-) content in the collected water samples in both seasons was found to be very low. For GW samples in the pre-monsoon period the range is Below Detectable Limit (BDL) -0.650 mgL-1 (mean 0.303 mgL-1) and in the post monsoon-season the range is BDL-0.710 mgL-1(mean 0.380 mgL-1). In India the maximum permitted value of fluoride in drinking water is 1.5 mgL-1. 21 So, in our studied area there is no risk for fluoride (F-) contaminated water and its health related issues.
Arsenic (As) content in water is a very important parameter to know especially for its suitability for drinking. In our present study, water samples collected in the pre-monsoon season were found to have Arsenic (As) content in variable amount from BDL-0.07 mgL-1(mean 0.007 mgL-1). Twelve numbers of samples (17.4%) were found to have Arsenic (As) content above permitted limit in pre-monsoon season. In post- monsoon, Arsenic (As) content in GW samples was found to fall in the range BDL to 0.056 mgL-1 (mean 0.0047 mgL-1). Only seven numbers (10.1%) of samples were found to have Arsenic (As) above WHO permissible limit of 0.01mgL-1. 24
The study clearly revealed that the concentration of most of the water quality constituents are under permissible limit of WHO, except the water samples have elevated concentration of iron, calcium and magnesium. Very few water samples have Arsenic (As) content above the permissible limit of WHO.
To know about the suitability of ground water samples for drinking, WQI of each water samples were evaluated for the wide-ranging depiction of ground water quality. It was calculated in three major steps following the method reported in literature. 10 IS specific for drinking water (BIS 1991), was applied for the computation of WQI.25 In the first step, different water parameters such as TDS , pH, TH, HCO3-, Cl-, SO42-, NO3-, F-, Ca2+, Mg2+, Fe, As etc were assigned weight (wi) in accordance with their importance in the water quality for drinking. Different weights assigned to each parameter based on their importance are as given in Table7. In second step, the following equation was applied to determined the relative weight (Wi) of each chemical parameter.
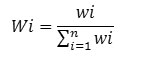
Where, the relative weight is represented Wi
Weight of each parameter is represented by wi
Total number of parameters is represented by n.
The computed relative weight (Wi) of each chemical parameter is shown in Table 7.
Table 7: Relative weight of Chemical Parameters
|
Chemical parameter1 |
Indian Standard2 |
Weight (wi) |
Relative weight (Wi)
|
|
pH |
6.5-8.5 |
4 |
0.0952 |
|
Total dissolved solid(TDS) |
500-2000 |
4 |
0.0952 |
|
Total Hardness(TH) |
300-600 |
2 |
0.0476 |
|
Bicarbonate (HCO3-) |
244-732 |
3 |
0.0714 |
|
Chloride (Cl-) |
250-1000 |
3 |
0.0714 |
|
Sulphate (SO42-) |
200-400 |
4 |
0.0952 |
|
Nitrate (NO3-) |
45-100 |
5 |
0.1190 |
|
Fluoride (F-) |
1-1.5 |
4 |
0.0952 |
|
Calcium (Ca2+) |
75-200 |
2 |
0.0476 |
|
Magnesium (Mg2+) |
30-100 |
2 |
0.0476 |
|
Iron(Fe) |
0.3-1.0 |
4 |
0.0952 |
|
Arsenic (As) |
0.01-0.05 |
5 |
0.1190 |
|
|
|
|
|
1 Chemical parameters are expressed in mg/L
2 Lower values signifies desirable limit, and higher value signifies permissible limit when there is no alternate source.25
In the final step, to calculate the quality rating scale (qi) for the parameters the following equation was used
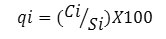
Here,
qi, the quality rating
Each chemical parameter’s concentration in each water sample is represented by Ci in mgL-1. IS of drinking water for each parameter is represented by Si in mgL-1.
For calculation of WQI, the sub index (SI) for each parameter is determined first according to the equation given below
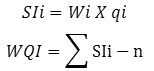
Here,
SIi , sub index of ith parameter;
Wi , relative weight of ith parameter.
qi ,is the rating calculated on the basis of concentration of ith parameter,
n , the number of chemical parameters.
WQI values are listed in the Table 8 and compared the data with the reported literature elsewhere .10 The WQI classification GW samples in both seasons are shown in Figure 2(a) and figure 2(b) respectively. From the figure it is observed that most of the water samples belong to poor category for drinking. The probable reason for this is the presence of elevated iron content in all water samples.
Table 8: Categorization of water samples for drinking on the basis of WQI
|
WQI Range |
Categorization |
Percentage of samples |
|
|
Pre |
post |
||
|
<50 |
Excellent |
1.5 |
1.5 |
|
50-100 |
Good |
19.1 |
14.7 |
|
100-200 |
Poor |
73.5 |
80.9 |
|
200-300 |
Very poor |
5.9 |
2.9 |
|
>300 |
Unfit for drinking |
0 |
0 |
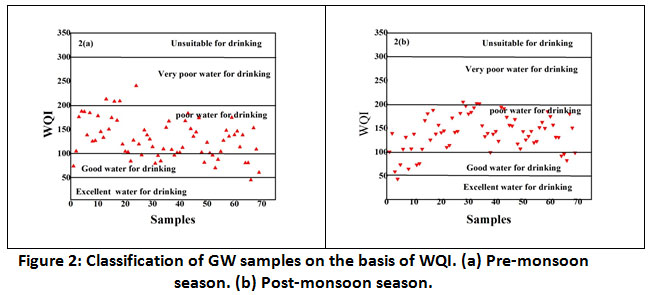 |
Figure 2: Classification of GW samples on the basis of WQI. (a) Pre-monsoon season. (b) Post-monsoon season. Click here to view Figure |
Classification of Ground Water for Irrigation
The parameters such as Electrical Conductivity (denoted as EC), Total Dissolved Solids (dented as TDS), Sodium Adsorption Ratio (denoted as SAR), Kelly’s Ratio (denoted as KR), Sodium Percent (Na %), Residual Sodium Carbonate (denoted as RSC), Magnesium Ratio (denoted as MR), and Corrosivity Ratio (denoted as CR) are adapted to find out the suitability of GW for irrigation.8
Elevated value of EC in GW causes salinity of soil. Richard (1954), classified the irrigation water in to five classes based on EC value shown in Table 9. 26 In our present study, all the water samples in both seasons were found be in excellent category.
SAR generally, expresses the sodium alkali hazard. Richards (1954), is used the following equation to calculate the values of SAR for each samples.26
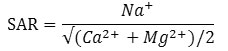
In this equation concentration of the cations are taken in meqL-1. Predominance of calcium and magnesium implies less alkali hazards but elevated concentration of sodium gives rise to high alkali hazards. SAR values of irrigation water have a notable relationship with the degree of sodium absorbed by the soil. Use of GW with elevated concentration of sodium for irrigation can increases the sodium content in soil which affect the soil texture and its permeability. Consequently, the soil becomes hard to plough and unfit for exposure of seedlings .27 The SAR values were found to be ranges from 0.16 to 1.07 during pre-monsoon and 0.113 to 1.15 during post-monsoon season. As, the SAR value in both season were found to be <10 for all the GW samples, so all the water samples belongs to excellent category for irrigation as per Richards (1954) classification.
The formula given below is used to calculate Sodium Percentage of water
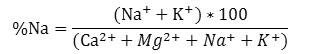
Where, the concentrations of all cations are taken in meqL-1. The sodium percentage was found to be varied in the range of 39.1 % to 73.6 % in pre- monsoon season and 31.3% to 73.4 % in the post- monsoon season. The outcomes of the results are listed in Table 9.
The ratio of sodium ion to calcium and magnesium ions is known as Kelly’s ratio (KR). The concentration of each ion is taken in meqL-1. The KR is calculated by following formula.
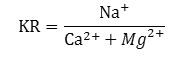
If KR is found to be greater than 1 for ground water, then it is considered as unfit for irrigation purposes. It is greater than 1 when concentration of Na+ is higher, in such situation the clay particle absorb Na+ ion and displaces Ca2+ and Mg2+ ions. This affects the internal drainage and results in the reduction of the permeability of the soil .28 In our studied GW samples, it was found that in the pre monsoon period 68.1 % samples are applicable for irrigation with KR value < 1 whereas in post monsoon period 87% water samples are applicable for irrigation with KR<1.
Magnesium ion to calcium and magnesium ion ratio is known as magnesium ratio (MR).29 It is expressed as
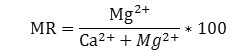
Concentration of each ion is expressed in meqL-1. Ground water having MR greater than 50% indicates suitability of water for irrigation purposes. When irrigation water contains higher concentration of Mg2+ content, it increases the alkalinity of the soil and decreases the crop production.30 In the current study, only 2.9 % water samples of pre-monsoon season were found to have MR values greater than 50% and are unsuitable for irrigation. Remaining water samples of both seasons were found to be applicable for irrigation on the basis of MR value with less than 50 %.
High percentage of HCO3- and CO32- in ground causes natural tendency for precipitation of Ca2+ and Mg2+ ions. To know the extent of this effect, Eaton (1950), proposed the term Residual Carbonate (RSC) which is calculated by the following formula. 31 The concentration of each ion taken in meqL-1. Lioyd and Heathcote (1985), categorize water based on RSC values.32 According to this categorization water samples with RSC below 1.25 are considered as appropriate for irrigation, whereas up to 2.5 RSC value, water is considered as marginally appropriate and above this water is unsuitable.
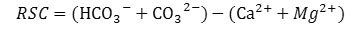
As per LIoyd and Heathcote (1985), classifications based on RSC value, 53.6% GW samples in pre-monsoon period were appropriate for irrigation and remaining 46.4 % water samples are marginally appropriate for irrigation.32 In post monsoon period all the water samples were found to be appropriate for irrigation having RSC value below 1.25.
The corrosivity ratio (CR) is calculated by the formula given below where concentration of each ion is expressed in meqL-1.
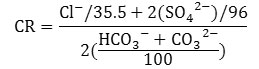
CR Values indicates the degree of corrosivity of ground water. For safe transportation of ground water by pipes the CR should be less than 1. CR value greater than 1 implies corrosive nature of water and unsafe for transportation by pipes.33 All the ground water samples in both seasons were ascertained to be suitable for transportation through pipes with CR value less than 1.
Table 9: Categorization of Ground water for irrigation purpose
|
Parameters |
Range |
Categorization |
Quantity of samples |
|
|
Pre monsoon |
Post monsoon |
|||
|
Salinity hazards (EC) (µS/cm)
|
<250 |
Excellent |
69 |
69 |
|
250-750 |
Good |
0 |
0 |
|
|
750-2000 |
Permissible |
0 |
0 |
|
|
2000-3000 |
Doubtful |
0 |
0 |
|
|
>3000 |
Inappropriate |
0 |
0 |
|
|
Total dissolved solid (TDS) |
<1000 |
Non saline |
69 |
69 |
|
1000-3000 |
Slightly Saline |
0 |
0 |
|
|
3000-10000 |
Reasonably saline |
0 |
0 |
|
|
>10000 |
Extremely Saline |
0 |
0 |
|
|
Sodium Percentage(Na%) |
<20 |
Excellent |
0 |
0 |
|
20-40 |
Good |
1 |
3 |
|
|
40-60 |
acceptable |
32 |
41 |
|
|
60-80 |
Doubtful |
36 |
25 |
|
|
>80 |
Inappropriate |
0 |
0 |
|
|
Alkalinity Hazard (SAR) |
<10 |
Excellent |
69 |
69 |
|
10-18 |
Good |
0 |
0 |
|
|
18-26 |
Doubtful |
0 |
0 |
|
|
>26 |
Inappropriate |
0 |
0 |
|
|
Residual Sodium carbonate (RSC) |
<1.25 |
appropriate |
37 |
69 |
|
>1.25-2.5 |
Marginally appropriate |
32 |
0 |
|
|
>2.5 |
Inappropriate |
0 |
0 |
|
|
Magnesium ratio (MR) |
>50% |
appropriate |
67 |
69 |
|
<50% |
Inappropriate |
2 |
0 |
|
|
Kellys ratio (KR) |
<1 |
appropriate |
47 |
60 |
|
>1 |
Inappropriate |
22 |
9 |
|
|
Corrosivity ratio (CR) |
<1 |
appropriate |
69 |
69 |
|
>1 |
Inappropriate |
0 |
0 |
|
US salinity Laboratory diagram (USSL) was also used to determine the class/type of water for irrigation purpose.34 USSL diagram is a plot of obtained values of EC (Salinity hazards) against SAR. In our present study, the diagram revealed that all the ground water samples of both season are C1-S1 water type (low salinity and low SAR) and are suitable for most of the crops on most of the soils. The USSL diagram is shown in figure 3(a) and 3(b).
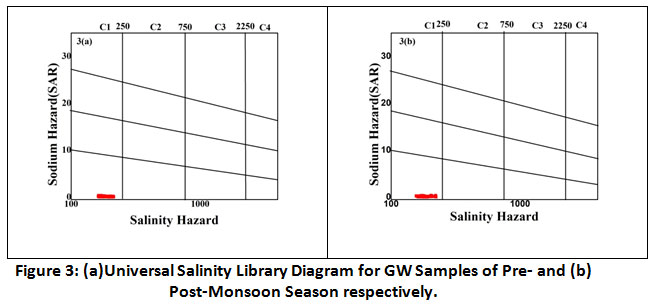 |
Figure 3: (a)Universal Salinity Library Diagram for GW Samples of Pre- and (b) Post-Monsoon Season respectively. Click here to view Figure |
Multivariate Statistical Analysis
Correlation Matrix Analysis
Correlation matrix analysis is a useful tool to know the extent of linear relationship among the significant physicochemical parameters and the primary cation and anion. The correlation matrix analysis study is presented in Table 10 and Table 11 for both pre and post monsoon seasons. For positive correlation between two parameters, the correlation coefficient (r) should be closure to +1 and when the correlation coefficient (r) closure to -1 it indicates negative linear correlation.35 Two parameters with positive correlation indicate their common source and negative correlation indicates that they are originated from different source. When r>0.50, the correlation is strong, for r=0.50 correlation is good and when it is less than 0.50 correlation is poor.
From the Table 9, it is observed that the pre-monsoon GW samples show strong positive correlation among the parameters like EC-TH, EC-Ca2+ , EC-Mg2+ , EC-TA , TDS-TH , TH-Ca2+ , TH-Mg2+, TH-TA, Ca2+-Mg2+, Ca2+-TA, Mg2+-TA. In addition to this, significant positive correlation was found among EC-TDS, EC-SO42-, TH-SO42-, TDS-Ca2+, TDS-Mg2+, TDS-TA, TDS-SO42-, Ca2+-SO42-, Mg2+- SO42-.
From the Table 10, it is observed that in the GW samples of post-monsoon season strong positive correlation was found among EC-TH , EC-Ca2+ , EC-Mg2+ ,TDS-TH , TH-Ca2+ , TH-Mg2+, TH-TA, Ca2+-Mg2+,. In addition to this, significant positive correlation was found among EC-TDS, EC-TA, EC-SO42-, TH-SO42-, TDS-Ca2+, TDS-Mg2+, TDS-TA, TDS-SO42-, Mg2+- SO42-. Ca2+-TA, Mg2+-TA.
Table 10: Correlation Matrix (Pre-monsoon Season)
|
Parameters |
pH |
EC |
TDS |
TA |
TH |
Na+ |
K+ |
Ca2+ |
Mg2+ |
Cl- |
SO42- |
F- |
|
pH |
1.000 |
|
|
|
|
|
|
|
|
|
|
|
|
EC |
.127 |
1.000 |
|
|
|
|
|
|
|
|
|
|
|
TDS |
.151 |
.975 |
1.000 |
|
|
|
|
|
|
|
|
|
|
TA |
.067 |
.835 |
.824 |
1.000 |
|
|
|
|
|
|
|
|
|
TH |
.106 |
.994 |
.974 |
.834 |
1.000 |
|
|
|
|
|
|
|
|
Na+ |
.087 |
.023 |
.044 |
-.004 |
.014 |
1.000 |
|
|
|
|
|
|
|
K+ |
.194 |
.081 |
.071 |
.080 |
.082 |
-.124 |
1.000 |
|
|
|
|
|
|
Ca2+ |
.099 |
.973 |
.955 |
.809 |
.976 |
.037 |
.075 |
1.000 |
|
|
|
|
|
Mg2+ |
.107 |
.985 |
.964 |
.830 |
.992 |
.000 |
.084 |
.941 |
1.000 |
|
|
|
|
Cl- |
.039 |
.113 |
.108 |
.083 |
.106 |
.083 |
-.206 |
.064 |
.128 |
1.000 |
|
|
|
SO42- |
.018 |
.856 |
.797 |
.688 |
.847 |
-.029 |
.076 |
.859 |
.822 |
.085 |
1.000 |
|
|
F- |
.095 |
.165 |
.126 |
.035 |
.161 |
-.108 |
-.037 |
.134 |
.173 |
.119 |
.154 |
1.000 |
Table 11: Correlation Matrix (Post-Monsoon Season)
|
Parameters |
pH |
EC |
TDS |
TA |
TH |
Na+ |
K+ |
Ca2+ |
Mg2+ |
Cl- |
SO42- |
F- |
|
pH |
1.000 |
|
|
|
|
|
|
|
|
|
|
|
|
EC |
.104 |
1.000 |
|
|
|
|
|
|
|
|
|
|
|
TDS |
.131 |
.963 |
1.000 |
|
|
|
|
|
|
|
|
|
|
TA |
.075 |
.784 |
.827 |
1.000 |
|
|
|
|
|
|
|
|
|
TH |
.151 |
.958 |
.979 |
.837 |
1.000 |
|
|
|
|
|
|
|
|
Na |
.037 |
.367 |
.403 |
.371 |
.461 |
1.000 |
|
|
|
|
|
|
|
K |
-.022 |
-.022 |
-.013 |
.060 |
-.015 |
-.055 |
1.000 |
|
|
|
|
|
|
Ca2+ |
.161 |
.946 |
.966 |
.827 |
.984 |
.443 |
.020 |
1.000 |
|
|
|
|
|
Mg2+ |
.143 |
.951 |
.973 |
.830 |
.995 |
.465 |
-.036 |
.961 |
1.000 |
|
|
|
|
Cl- |
.232 |
-.063 |
-.072 |
-.103 |
-.107 |
-.048 |
-.003 |
-.113 |
-.102 |
1.000 |
|
|
|
SO42- |
.069 |
.440 |
.475 |
.400 |
.488 |
.278 |
.100 |
.516 |
.465 |
-.184 |
1.000 |
|
|
F- |
.041 |
.153 |
.142 |
.076 |
.108 |
.099 |
.018 |
.140 |
.087 |
.244 |
-.101 |
1.000 |
Conclusion
The quality assessment of the GW samples revealed, that most of the water samples have physico-chemical parameters within the acceptable range of BIS (2012) for drinking. So, on the basis of physico-chemical parameters the water samples can be considered as suitable for domestic uses. From the WQI, it was observed that 1.5 % of the collected water samples were found to be excellent, 19.1 % good, 73.5 % poor, and 5.9 % to be of very poor quality for drinking in the pre-monsoon season. Similarly, in the post-monsoon season WQI showed that 1.5 % of water samples were excellent, 14.7 % good, 80.9 % poor and only 2.9 % of water samples were found to be very poor for drinking .The probable reason for poor WQI in most of the water samples is due to the presence of more iron than the permissible limit. It was classified on the basis of sodium percentage that 46.3 % of water samples in pre-monsoon and 36.2% of samples in post monsoon season were doubtful for irrigation. RSC values indicated that 46.3 % water samples in pre-monsoon periods were marginally appropriate for irrigation and in post-monsoon period all the samples were appropriate. The MR values indicated that 2.9 % samples in pre-monsoon period inappropriate for irrigation. From the KR values it was classified that 31.8 % samples in pre-monsoon period and 13.04 % samples in post-monsoon period were inappropriate for irrigation. The values of other parameters such as TDS, EC, SAR, and CR indicated that all water samples in both season were appropriate for irrigation for most of the crops. The contaminant like fluoride was found absent in all the water samples. So, ground water samples were found safe for drinking on the basis of fluoride contamination. Accordingly, it may be stated the area where the limited study was carried out is relatively free from these toxic contaminants. However, to have a full proof conclusion a thorough and round the year monitoring with wider sets of data collection is extremely important. Arsenic was found in few GW samples in both seasons. In pre-monsoon season 17.3 % water samples have Arsenic (As) above permissible limit and in post monsoon season 10.14 % water samples have Arsenic (As) above permissible limit.
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Acknowledgements
The authors are thankful to the Director, CSIR-NEIST, Jorhat, India for allowing to carry out the work .The authors are also thankful to Chemistry Department, Gauhati University, Assam, India for giving the opportunity to register under Ph. D programme.
References
- Meride Y, Ayenew B. Drinking water quality assessment and its effects on residents health in Wondo genet campus, Ethiopia. Environ Syst Res 2016; 5(1). DOI 10.1186/s40068-016-0053-6.
CrossRef - Okello C, Bruno T, Greggio N, Wambiji N, Antonellini M. Impact of Population Growth and Climate Change on the Freshwater Resources of Lamu Island, Kenya. Water 2015;7(3):1264-1290.
CrossRef - Kaur T, Bhardwaj R, Arora S. Assessment of groundwater quality for drinking and irrigation purposes using hydrochemical studies in Malwa region, southwestern part of Punjab, India. Appl. Water Sci. 2017; 7,3301–3316.
CrossRef - Ashbolt N. J. Microbial contamination of drinking water and disease outcomes in developing regions. Toxicology 2004; 198 (1-3):229–238.
CrossRef - Chetia M, Chatterjee S, Banerjee S, Nath M. J., Singh L, Srivastava R. B., Sarma H. P. Groundwater arsenic contamination in Brahmaputra river basin: a water quality assessment in Golaghat (Assam), India. Environ Mmonit Assessment. 2010; 173(1-4):371-85.
CrossRef - Jain C.K., Bandyopadhyay A, Bhadra A. Assessment of ground water quality for drinking purpose, District Nainital, Uttarakhand. India. Environ Monit Assessment.2010; 166,663-676.
CrossRef - Kundu A, Nag S. K. Assessment of groundwater quality in Kashipur Block, Purulia district, WestBengal. Appl. Water Sci. 2018; 8, 33. https://doi.org/10.1007/s13201-018-0675-0.
CrossRef - Jain C.K., Vaid U. Assessment of ground water quality for drinking and irrigation purposes using hydrochemical studies in Nalbari district of Assam, India. Environ. Earth sci. 2018; 77,254.
CrossRef - Madhava S, Ahamad A, Kumar A, Kushawaha J, Singh P, Mishra P. K. Geochemical assessment of groundwater quality for its suitability for drinking and irrigation purpose in rural areas of Sant Ravidas Nagar (Bhadohi), Uttar Pradesh. Geology, Ecology, and Landscapes. 2018; 2(2):127–136.
CrossRef - Batabyal A.K., Chakraborty S. Hydrogeochemistry and Water Quality Index in the Assessment of Groundwater Quality for Drinking Uses. WER 2015; 87 (7):607-617.
CrossRef - APHA, Awwa (American Public Health Association) (1988) standard methods for the examination of water and wastewater. American Public Health Association, Washington DC.
- World Health Organisation (WHO)(2011) Guidelines for drinking water quality , 4th edn. World Health Organisation, Geneva.
- Khound N.J., Bhattacharyya K.G. Assessment of water quality in and around Jia‑Bharali river basin, North Brahmaputra Plain, India, using multivariate statistical technique. Appl. Water Sci. 2018; 8, 221.
CrossRef - WHO (World Health Organisation ) (1984) Guidelines for drinking water quality, vol 2.Health crieteria and other supporting information WHO Publ., Geneva, p 335.
- Davis S, DeWies,t R.M. Hydrogeology. Wiley, New York (1966).
- Durfor C.N., Becker E. Publick water supplies of 100 largest cities in the United State, 1962. Geological Survey. Water supply paper. 1964; 1812. doi: https://doi.org/10.3133/wsp1812.
CrossRef - Bidhuri S, Khan M.M.A. Assessment of Ground water Quality of Central and Southeast District of NCT of Delhi. Jour.Geological Society of India.2020; 95,95-103.
CrossRef - Kumar P.J.S., James E.J. Physicochemical parameters and their sources in groundwater in the Thirupathur region, Tamil Nadu, South India. Appl Water Sci.2013; 3,219–228 DOI 10.1007/s13201-012-0074-x.
CrossRef - IS 10500: 1991 Drinking Water-Specification (First Revision).
- Pal S.K., Rajpaul, Ramprakash, Bhat, M.A., Yadav S.S. Assessment of ground water quality for irrigation use in Firozpur-jhirka block in Mewat district of Haryana, north India. JSSWQ .2018; 10(2):157-167.
- IS 10500: 2012 Drinking Water-Specification(Second Revision).
- Mariappan P., Yegnaraman V., Vasudeva T. Groundwater quality fluctuation with water table in Thiruppathur block of Sivagangai district, Tamil Nadu. Pollut. Res.. 2000; 19(2):225–229.
- Fewtrel L. Drinking-Water Nitrate, Methemoglobinemia, and Global Burden of Disease: A Discussion. Environ Health Prospect 2004;112(14):1371-1374.
CrossRef - WHO (World Health Organisation) (2017) Guidelines for drinking-water quality: fourth edition incorporating the first addendum ISBN 978-92-4-154995-0.
- Bureau of Indian Standards (1991) Indian Standard Drinking Water Specification. 1st rev. Bureau of Indian Standards: New Dehli, India.
- Richards L.A. (1954). Diagnosis and Improvement of Saline and Alkali Soils. Agricultural Handbook No. 60. United States Department of Agriculture, Washington DC.
CrossRef - Keesari T., Ramakumar K.L., Chidambaram S., Pethperumal S., Thilagavathi R. Understanding the hydrochemical behavior of groundwater and its suitability for drinking and agricultural purposes in Pondicherry area, South India—a step towards sustainable development. Ground w. for Sustain. Dev.2016; 23,143–153.
CrossRef - Karnath K.R., Groundwater assessment, development and management1987, Tata-MCGRAW-Hill, New Delhi.
- Szabolcs I., Darab C. The influence of irrigation water of high sodium carbonate of soils. In: Proceedings of 8th international congress of ISSS, transmission.1964;2:803-812
- Kaur, T., Bhardwaj R., Arora S. Assessment of groundwater quality for drinking and irrigation purposes using hydrochemical studies in Malwa region, southwestern part of Punjab, India. Appl Water Sci. 2017; 7:3301–3316.
CrossRef - Eaton F.M., Significance of carbonates in irrigation waters. Soil Sci.1950;69;2:123-134.
CrossRef - LIoyd J.W., Heathcote J.A.,Natural inorganic hydrochemistry in relation to ground water . Clarendon , Oxford.1985; 294.
- Mishra U.K., Tripathi A.K. ,Tiwari S.,Mishra A. Assessment of Quality and Pollution Potential of Groundwater around Dabhaura Area, Rewa District, Madhya Pradesh, India. Earth Science Research.2012;1(2):249-261.
CrossRef - Keesari T., Sinha U.K., Kamaraj P., Sharma D.A. Groundwater quality in a semi-arid region of India: Suitability for drinking , agriculture and fluoride exposure risk .J.Earth Sys.Sci..2019;128:1-24.
CrossRef - Sukanya S.,Joseph S. Water Quality Assessment using Environmetrics and Pollution Indices in A Tropical River, Kerala, SW Coast of India. Curr. World Environ.2020;
CrossRef